Description
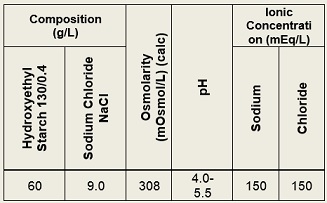
HydroStarch™ is a sterile, non-pyrogenic solution as an aid for hypovolemia. May be administered via intravenous infusion using aseptic technique. It contains no antimicrobial agents. Discard any unused portion. Composition, osmolarity, pH and ionic concentration are shown in Table 1.
Table 1
The container is free of PVC and phthalates. The container meets the requirements of USP and is registered with FDA.
Clinical Pharmacology
HydroStarch™ contains hydroxyethyl starch in a colloidal solution which expands plasma volume when administered intravenously. Hydroxyethyl starch is a derivative of thin boiling waxy corn starch, which mainly consists of a glucose polymer (amylopectin). Substitution of hydroxyethyl groups on the glucose units of the polymer reduces the normal degradation of amylopectin by α-amylase in the body.
Indications
HydroStarch™ act as a plasma volume substitute for the treatment and prophylaxis of hypovolemia in all species. It is not a substitute for red blood cells or coagulation factors in plasma.
Contraindications
HydroStarch™ is contraindicated in patients with a known hypersensitivity to hydroxyethyl starch, fluid overload (hyperhydration) and especially in cases of pulmonary edema and congestive heart failure, renal failure with oliguria or anuria not related to hypovolemia, patients receiving dialysis treatment, severe hypernatremia or severe hyperchloremia and intracranial bleeding.
Warnings
Anaphylactoid reactions (bradycardia, tachycardia, bronchospasm, non-cardiac pulmonary edema) have been reported with solutions containing hydroxyethyl starch. If a hypersensitivity reaction occurs, administration of the drug should be discontinued immediately and the appropriate treatment and supportive measures should be undertaken until symptoms have resolved.
Fluid status and rate of infusion should be assessed regularly during treatment, especially in patients with cardiac insufficiency or severe kidney dysfunction.
In cases of severe dehydration, a crystalloid solution should be given first. Generally, sufficient fluid should be administered in order to avoid dehydration.
Caution should be observed before administering HydroStarch™ to patients with severe liver disease or severe bleeding disorders. With the administration of certain hydroxyethyl starch solutions, disturbances of blood coagulation can occur depending on the dosage.
If administered by pressure infusion, air should be withdrawn or expelled from the bag through the administration port prior to infusion.
Do not introduce additives into this container.
Adverse Reactions
Products containing hydroxyethyl starch may lead to anaphylactoid reactions (hypersensitivity, mild influenza-like symptoms, bradycardia, tachycardia, bronchospasm, non-cardiac pulmonary edema).
Prolonged administration of high dosages of hydroxyethyl starch may cause pruritus (itching), an undesirable effect observed with all hydroxyethyl starches.
At high doses, the dilutional effects may result in decreased levels of coagulation factors and other plasma proteins, and a decreased in hematocrit.
If an adverse reaction does occur, discontinue the infusion and evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
Precautions
This is a single dose unit. It contains no preservatives. Use entire contents when first opened.
Do not administer unless solution is clear and seal is intact.
Solution must be warmed to body temperature prior to administration and administered at a slow rate. Use solution promptly following initial entry.
Reactions which may occur because of the solution or the technique of administration, include febrile response, infection at the site of injection, and extravasation.
Drug Interactions
No interactions with other drugs or nutritional products are known. The safety and compatibility of other additives have not been established.
Dosage and Administration
To be used as directed by a licensed veterinarian. The dosage of the HydroStarch™ is dependent upon the blood loss, hemodynamics and on the hemodilution effects of the patient. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
For use in one patient on one occasion only. Discard any unused portion. Care should be taken with administration technique to avoid administration site reactions and infection.
HydroStarch™ can be administered repetitively over several days. The initial 10 to 20mL should be infused slowly, keeping the patient under close observation due to possible anaphylactoid reaction. See Warnings and Precautions.
Over-dosage
As with all plasma volume substitutes, over-dosage can lead to overloading of the circulatory system (e.g. pulmonary edema). In this case, the infusion should be stopped immediately and, if necessary, a diuretic should be administered. See Warnings, Precautions and Adverse Reactions.
Storage
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended that the product be stored at 59 to 77°F (15 to 25°C). Protect from freezing.
Directions for use of plastic container
To Open
Tear overwrap at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing solution container firmly. If leaks are found, discard solution as sterility may be impaired. If supplemental medication is desired, follow directions below:
Preparation for Administration
1. Suspend container from eyelet support.
2. Remove plastic protector from inlet/outlet port at bottom of container.
3. Attach administration set.
WARNING: Do not introduce additives into this container.
CAUTION: FEDERAL LAW RESTRICTS THIS DRUG TO USE BY OR ON THE ORDER OF A LICENSED VETERINARIAN.

