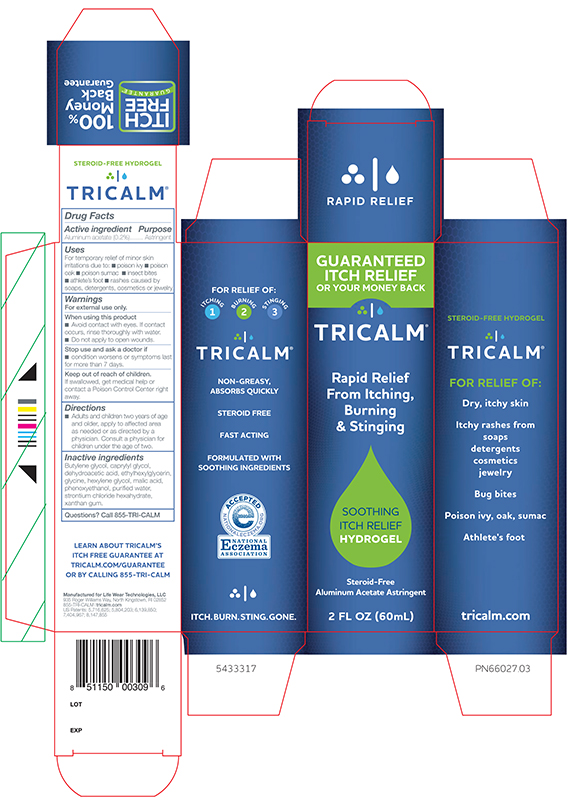
TRICALM HYDROGEL- aluminum acetate gel
Modular Thermal Technologies LLC Dba Life Wear Technologies
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Aluminum acetate (0.2%)
Uses
For temporary relief of minor skin irritations due to:
- poison ivy
- poison oak
- poison sumac
- insect bites
- athlete's foot
- rashes caused by soaps, detergents, cosmetics or jewelry
Warnings
For external use only.
When using this product
- Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
- Do not apply to open wounds.
Stop use and ask a doctor if
- condition worsens or symptoms last for more than 7 days.
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children two years of age and older, apply to affected area as needed or as directed by a physician.
- Consult a physician for children under the age of two.
Inactive ingredients
Butylene glycol, caprylyl glycol, dehydroacetic acid, ethylhexylglycerin, glycine, hexylene glycol, malic acid, phenoxyethanol, purified water, strontium chloride hexahydrate, xanthan gum.
Questions?
Call 855-TRI-CALM
PRINCIPAL DISPLAY PANEL - 60 mL Vertical Carton
TRICALM®
Rapid Relief
From Itching,
Burning
& Stinging
SOOTHING
ITCH RELIEF
HYDROGEL
Steroid-Free
Aluminum Acetate Astringent
2 FL OZ (60mL)
Principal Display Panel Tube
TRICALM Steroid-Free Hydrogel
For Itching Burning and Stinging
2.0 FLOZ ( 60mL)



