OPANA ER contains oxymorphone, which is a morphine-like opioid agonist and a Schedule II controlled substance, with an abuse liability similar to other opioid analgesics.
Oxymorphone can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing OPANA ER in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion.
OPANA ER is an extended-release oral formulation of oxymorphone indicated for the management of moderate to severe pain when a continuous, around-the-clock opioid analgesic is needed for an extended period of time.
OPANA ER is NOT intended for use as a prn analgesic.
OPANA ER Tablets are to be swallowed whole and are not to be broken, chewed, dissolved, or crushed. Taking broken, chewed, dissolved, or crushed OPANA ER Tablets leads to rapid release and absorption of a potentially fatal dose of oxymorphone.
Patients must not consume alcoholic beverages, or prescription or non-prescription medications containing alcohol, while on OPANA ER therapy. The co-ingestion of alcohol with OPANA ER may result in increased plasma levels and a potentially fatal overdose of oxymorphone.
DESCRIPTION
OPANA ER (oxymorphone hydrochloride) extended-release, is a semi-synthetic opioid analgesic supplied in 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for oral administration. The tablet strength describes the amount of oxymorphone hydrochloride per tablet. The tablets contain the following inactive ingredients: hypromellose, iron oxide black, methylparaben, propylene glycol, silicified microcrystalline cellulose, sodium stearyl fumarate, TIMERx® -N, titanium dioxide, and triacetin. The 5 mg, 10 mg and 20 mg tablets also contain macrogol, and polysorbate 80. In addition, the 5 mg tablets contain iron oxide red. The 10 mg tablets contain FD and C yellow No. 6. The 20 mg tablets contain FD and C blue No. 1, FD and C yellow No. 6, and D and C yellow No. 10. The 40 mg tablets contain FD and C yellow No. 6, D and C yellow No. 10, and lactose monohydrate.
Chemically, oxymorphone hydrochloride is 4, 5α -epoxy-3, 14-dihydroxy-17-methylmorphinan-6-one hydrochloride, a white or slightly off-white, odorless powder, which is sparingly soluble in alcohol and ether, but freely soluble in water. The molecular weight of oxymorphone hydrochloride is 337.80. The pKa1 and pKa2 of oxymorphone at 37°C are 8.17 and 9.54, respectively. The octanol/aqueous partition coefficient at 37°C and pH 7.4 is 0.98.
The structural formula for oxymorphone hydrochloride is as follows:
The tablet strengths, 5, 10, 20 and 40 mg, describe the amount of oxymorphone hydrochloride per tablet.
CLINICAL PHARMACOLOGY
Oxymorphone is an opioid agonist whose principal therapeutic action is analgesia. Other members of the class known as opioid agonists include substances such as morphine, oxycodone, hydromorphone, fentanyl, codeine, hydrocodone, and tramadol. In addition to analgesia, other pharmacological effects of opioid agonists include anxiolysis, euphoria, feelings of relaxation, respiratory depression, constipation, miosis, and cough suppression. Like all pure opioid agonist analgesics, with increasing doses there is increasing analgesia, unlike with mixed agonist/antagonists or non-opioid analgesics, where there is a limit to the analgesic effect with increasing doses. With pure opioid agonist analgesics, there is no defined maximum dose; the ceiling to analgesic effectiveness is imposed only by side effects, the more serious of which may include somnolence and respiratory depression.
Central Nervous System
The precise mechanism of the analgesic action is unknown. However, specific CNS (central nervous system) opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and play a role in the analgesic effects of this drug. In addition, opioid receptors have also been identified within the PNS (peripheral nervous system). The role that these receptors play in these drugs’ analgesic effects is unknown.
Opioids produce respiratory depression likely by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increases in carbon dioxide tension and electrical stimulation.
Opioids depress the cough reflex by direct effect on the cough center in the medulla oblongata. Antitussive effects may occur with doses lower than those usually required for analgesia. Opioids cause miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations (see OVERDOSAGE: Signs and Symptoms).
Gastrointestinal Tract and Other Smooth Muscle
Opioids cause a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid-induced effects may include a reduction in gastric, biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Cardiovascular System
Opioids produce peripheral vasodilation which may result in orthostatic hypotension. Release of histamine can occur and may contribute to opioid-induced hypotension. Manifestations of histamine release may include orthostatic hypotension, pruritus, flushing, red eyes, and sweating. Animal studies have shown that oxymorphone has a lower propensity to cause histamine release than other opioids.
Endocrine System
Opioids have been shown to have a variety of effects on the secretion of hormones. Opioids inhibit the secretion of ACTH, cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon in humans and other species, rats and dogs. Thyroid stimulating hormone (TSH) has been shown to be both inhibited and stimulated by opioids.
Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown.
Pharmacodynamics
Concentration-Efficacy RelationshipsStudies in healthy volunteers reveal predictable relationships between OPANA ER dosage and plasma oxymorphone concentrations.
The minimum effective plasma concentration of oxymorphone for analgesia varies widely among patients, especially among patients who have been previously treated with agonist opioids. As a result, patients need to be individually titrated to achieve a balance between therapeutic and adverse effects. The minimum effective analgesic concentration of oxymorphone for any individual patient may increase over time due to an increase in pain, progression of disease, development of a new pain syndrome and/or potential development of analgesic tolerance.
Concentration-Adverse Experience RelationshipsOPANA ER is associated with typical opioid-related adverse experiences. There is a general relationship between increasing opioid plasma concentration and increasing frequency of adverse experiences such as nausea, vomiting, CNS effects, and respiratory depression.
As with all opioids, the dose must be individualized (see DOSAGE AND ADMINISTRATION). The effective analgesic dose for some patients will be too high to be tolerated by other patients.
Pharmacokinetics
AbsorptionThe absolute oral bioavailability of oxymorphone is approximately 10%.
Steady-state levels are achieved after three days of multiple dose administration. Under both single-dose and steady-state conditions, dose proportionality has been established for the 5 mg, 10 mg, 20 mg, and 40 mg tablet strengths for both peak plasma levels (Cmax) and extent of absorption (AUC) (Table 1).
Table 1: Mean (+SD) OPANA ER Pharmacokinetic Parameters
| Regimen | Dosage | Cmax
(ng/mL) | AUC (ng·hr/mL) | T ½
(hr) |
| Single Dose |
5 mg |
0.27±0.13 |
4.54±2.04 |
11.30±10.81
|
| Multiple Dosea |
5 mg |
0.70±0.55 |
5.60±3.87 |
NA |
a Results after 5 days of every 12 hours dosing.
Food Effect
Two studies examined the effect of food on the bioavailability of single doses of 20 and 40 mg of OPANA ER in healthy volunteers. In both studies, after the administration of OPANA ER, the Cmax was increased by approximately 50% in fed subjects compared to fasted subjects. A similar increase in Cmax was also observed with oxymorphone solution.
The AUC was unchanged in one study and increased by approximately 18% in the other study in fed subjects following the administration of OPANA ER. Examination of the AUC suggests that most of the difference between fed and fasting conditions occurs in the first four hours after dose administration. After oral dosing with a single dose of 40 mg, a peak oxymorphone plasma level of 2.8 ng/ml is achieved at 1 hour in fasted subjects and a peak of 4.25 ng/ml is achieved at 2 hours in fed subjects and that beyond the 12 hour time point, there is very little difference in the curves. As a result, OPANA ER should be dosed at least one hour prior to or two hours after eating (see DOSAGE AND ADMINISTRATION).
Ethanol Effect
In Vivo OPANA ER Formulation-Alcohol Interaction
Although in vitro studies have demonstrated that OPANA ER does not release oxymorphone more rapidly in 500 mL of 0.1N HCl solutions containing ethanol (4%, 20%, and 40%), there is an in vivo interaction with alcohol. An in vivo study examined the effect of alcohol (40%, 20%, 4% and 0%) on the bioavailability of a single dose of 40 mg of OPANA ER in healthy, fasted volunteers. The results showed that the oxymorphone mean AUC was 13% higher (not statistically significant) after co-administration of 240 mL of 40% alcohol. The AUC was essentially unaffected in subjects following the co-administration of OPANA ER and ethanol (240 mL of 20% or 4% ethanol).
There was a highly variable effect on Cmax with concomitant administration of alcohol and OPANA ER. The change in Cmax ranged from a decrease of 50% to an increase of 270% across all conditions studied. Following concomitant administration of 240 mL of 40% ethanol the Cmax increased on average by 70% and up to 270% in individual subjects. Following the concomitant administration of 240 mL of 20% ethanol, the Cmax increased on average by 31% and up to 260% in individual subjects. Following the concomitant administration of 240 mL of 4 % ethanol, the Cmax increased 7% on average and by as much as 110% for individual subjects. After oral dosing with a single dose of 40 mg in fasted subjects, the mean peak oxymorphone plasma level is 2.4 ng/mL and the median Tmax is 2 hours. Following co-administration of OPANA ER and alcohol (240 mL of 40% ethanol) in fasted subjects, the mean peak oxymorphone level is 3.9 ng/mL and the median Tmax is 1.5 hours (range 0.75 – 6 hours).
Co-administration of oxymorphone and ethanol must be avoided.
Oxymorphone may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression because respiratory depression, hypotension, and profound sedation, coma, or death may result.
Distribution
Formal studies on the distribution of oxymorphone in various tissues have not been conducted. Oxymorphone is not extensively bound to human plasma proteins; binding is in the range of 10% to 12%.
Metabolism
Oxymorphone is highly metabolized principally in the liver and undergoes reduction or conjugation with glucuronic acid to form both active and inactive metabolites. The two major metabolites of oxymorphone are oxymorphone-3-glucuronide and 6-OH-oxymorphone. The mean plasma AUC for oxymorphone-3-glucuronide is approximately 90-fold higher than the parent compound. The pharmacologic activity of the glucuronide metabolite has not been evaluated. 6-OH-oxymorphone has been shown in animal studies to have analgesic bioactivity. The mean plasma 6-OH-oymorphone AUC is approximately 70% of the oxymorphone AUC following single oral doses, but is essentially equivalent to the parent compound at steady-state.
Excretion
Because oxymorphone is extensively metabolized, less than 1% of the administered dose is excreted unchanged in the urine. On average, 33% to 38% of the administered dose is excreted in the urine as oxymorphone-3-glucuronide and 0.25% to 0.62% excreted as 6-OH-oxymorphone in subjects with normal hepatic and renal function. In animals given radiolabeled oxymorphone, approximately 90% of the administered radioactivity was recovered within 5 days of dosing. The majority of oxymorphone-derived radioactivity was found in the urine and feces.
Special Populations
Elderly
The steady-state plasma concentrations of oxymorphone, 6-OH-oxymorphone, and oxymorphone-3-glucuronide are approximately 40% higher in elderly subjects (≥ 65 years of age) than in young subjects (18 to 40 years of age). On average, age greater than 65 years was associated with a 1.4-fold increase in oxymorphone AUC and a 1.5-fold increase in Cmax. This observation does not appear related to a difference in body weight, metabolism, or excretion of oxymorphone (see PRECAUTIONS: Geriatric Use).
Gender
The effect of gender was evaluated following single- and multiple-doses of OPANA ER in male and female adult volunteers. There was a consistent tendency for female subjects to have slightly higher AUCss and Cmax values than male subjects; however, gender differences were not observed when AUCss and Cmax were adjusted by body weight.
Hepatic Impairment
The liver plays an important role in the pre-systemic clearance of orally administered oxymorphone. Accordingly, the bioavailability of orally administered oxymorphone may be markedly increased in patients with moderate to severe liver disease. The disposition of oxymorphone was compared in 6 patients with mild, 5 patients with moderate, and one patient with severe hepatic impairment and 12 subjects with normal hepatic function. The bioavailability of oxymorphone was increased by 1.6-fold in patients with mild hepatic impairment and by 3.7-fold in patients with moderate hepatic impairment. In one patient with severe hepatic impairment, the bioavailability was increased by 12.2-fold. The half-life of oxymorphone was not significantly affected by hepatic impairment (see DOSAGE AND ADMINISTRATION: Patients with Hepatic Impairment).
Renal Impairment
Data from a pharmacokinetic study involving 24 patients with renal dysfunction show an increase of 26%, 57%, and 65% in oxymorphone bioavailability in mild (creatinine clearance 51-80 mL/min; n=8), moderate (creatinine clearance 30-50 mL/min; n=8), and severe (creatinine clearance less than 30 mL/min; n=8) patients, respectively, compared to healthy controls.
Drug-Drug Interactions
In vitro studies revealed little to no biotransformation of oxymorphone to 6-OH-oxymorphone by any of the major cytochrome P450 (CYP P450) isoforms at therapeutically relevant oxymorphone plasma concentrations.
No inhibition of any of the major CYP P450 isoforms was observed when oxymorphone was incubated with human liver microsomes at concentrations of ≤ 50 μM. An inhibition of CYP3A4 activity occurred at oxymorphone concentrations ≥150 µM. Therefore, it is not expected that oxymorphone, or its metabolites will act as inhibitors of any of the major CYP P450 enzymes in vivo.
Increases in the activity of the CYP 2C9 and CYP 3A4 isoforms occurred when oxymorphone was incubated with human hepatocytes. However, clinical drug interaction studies with OPANA ER showed no induction of CYP450 3A4 or 2C9 enzyme activity, indicating that no dose adjustment for CYP 3A4- or 2C9-mediated drug-drug interactions is required.
CLINICAL TRIALS
The efficacy and safety of OPANA ER have been evaluated in double-blind, controlled clinical trials in opioid-naïve and opioid-experienced patients with moderate to severe pain including low back pain.
12-Week Study in Opioid-Naïve Patients with Low Back Pain
Patients with chronic low back pain who were suboptimally responsive to their current non-opioid therapy entered a 4-week, open-label dose titration phase. Patients initiated therapy with two days of treatment with OPANA ER 5 mg, every 12 hours. Thereafter, patients were titrated to a stabilized dose, at increments of 5-10 mg every 12 hours every 3-7 days. Of the patients who were able to stabilize within the Open-Label Titration Period, the mean±SD VAS score at Screening was 69.4±11.8 mm and at Baseline (beginning of Double-Blind Period) were 18.5±11.2 mm and 19.3±11.3 mm for the oxymorphone ER and placebo groups, respectively. Sixty three percent of the patients enrolled were able to titrate to a tolerable dose and were randomized into a 12-week double-blind treatment phase with placebo or their stabilized dose of OPANA ER. The mean±SD stabilized doses were 39.2±26.4 mg and 40.9±25.3 mg for the OPANA ER and placebo groups, respectively; total daily doses ranged from 10-140 mg. During the first 4 days of double-blind treatment patients were allowed an unlimited number of OPANA, an immediate-release (IR) formulation of oxymorphone, 5 mg tablets, every 4-6 hours as supplemental analgesia; thereafter the number of OPANA was limited to two tablets per day. This served as a tapering method to minimize opioid withdrawal symptoms in placebo patients. Sixty-eight percent of patients treated with OPANA ER completed the 12-week treatment compared to forty seven percent of patients treated with placebo. OPANA ER provided superior analgesia compared to placebo. The analgesic effect of OPANA ER was maintained throughout the double-blind treatment period in 89% of patients who completed the study. These patients reported a decrease, no change, or a ≤10 mm increase in VAS score from Day 7 until the end of the study.
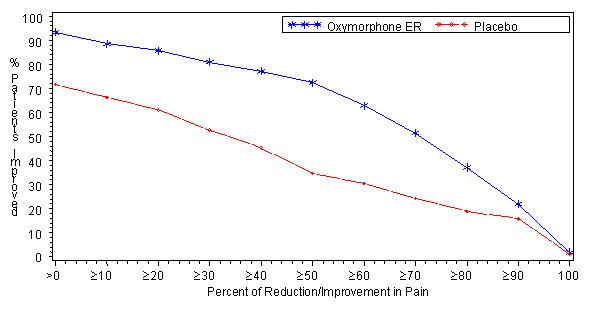
A significantly higher proportion of OPANA ER patients (81.4%) had at least a 30% reduction in pain score from screening to study endpoint compared to placebo patients (51.7%). The proportion of patients with various degrees of improvement from screening to study endpoint is shown in Figure 1.
Figure 1: Percent Reduction in Average Pain Intensity from Screening to Final Visit
12-Week Study in Opioid-Experienced Patients with Low
Back Pain
Patients currently on chronic opioid therapy entered a 4-week, open-label titration phase with OPANA ER dosed every 12 hours at an approximated equianalgesic dose of their pre-study opioid medication. Of the patients who were able to stabilize within the Open‑Label Titration Period, the mean±SD VAS score at Screening was 69.5±17.0 mm and at Baseline (beginning of Double-Blind Period) were 23.9±12.1 mm and 22.2±10.8 mm for the oxymorphone ER and placebo groups, respectively. Stabilized patients entered a 12‑week double-blind treatment phase with placebo or their stabilized dose of OPANA ER. The mean±SD stabilized doses were 80.9±59.3 mg and 93.3±61.3 mg for the OPANA ER and placebo groups, respectively; total daily doses ranged from 20-260 mg. During the first 4 days of double-blind treatment, patients were allowed an unlimited number of OPANA 5 mg tablets, every 4-6 hours as supplemental analgesia; thereafter the number of OPANA was limited to two tablets per day. This served as a tapering method to minimize opioid withdrawal symptoms in placebo patients. Fifty seven percent of patients were titrated to a stabilized dose within approximately 4 weeks of OPANA ER dose titration. Seventy percent of patients treated with OPANA ER and 26% of patients treated with placebo completed the 12-week treatment. OPANA ER provided superior analgesia compared to placebo . The analgesic effect of OPANA ER was maintained throughout the double-blind treatment period in 80 % of patients who completed the study. These patients reported a decrease, no change, or a ≤10 mm increase in VAS score from Day 7 until the end of the study.
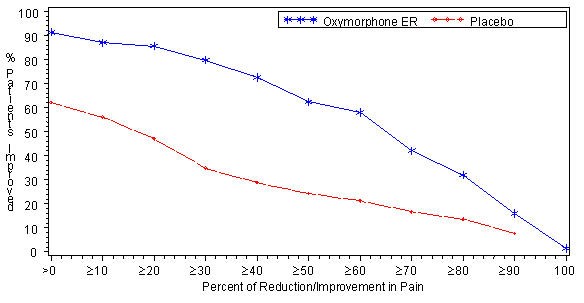
A significantly higher proportion of OPANA ER patients (79.7%) had at least a 30% reduction in pain score from screening to study endpoint compared to placebo patients (34.8%). Proportion of patients with various degrees of improvement from screening to study endpoint is shown in Figure 2.
Figure 2: Percent Reduction in Average Pain Intensity from Screening to Final Visit
INDICATIONS AND USAGE
OPANA ER is indicated for the relief of moderate to severe pain in patients requiring continuous, around-the-clock opioid treatment for an extended period of time.
OPANA ER is not intended for use as a prn analgesic.
OPANA ER is not indicated for pain in the immediate post-operative period (12-24 hours following surgery) for patients not previously taking opioids because of the risk of oversedation and respiratory depression requiring reversal with opioid antagonists.
OPANA ER is not indicated for pain in the post-operative period if the pain is mild or not expected to persist for an extended period of time.
CONTRAINDICATIONS
OPANA ER is contraindicated in patients with a known hypersensitivity to oxymorphone hydrochloride or to any of the other ingredients in OPANA ER, or with known hypersensitivity to morphine analogs such as codeine.
OPANA ER is not indicated for pain in the immediate post-operative period (the first 12-24 hours following surgery), or if the pain is mild, or not expected to persist for an extended period of time. OPANA ER is only indicated for post-operative use if the patient is already receiving the drug prior to surgery or if the post-operative pain is expected to be moderate or severe and persist for an extended period of time. Physicians should individualize treatment, moving from parenteral to oral analgesics as appropriate. (See American Pain Society guidelines).
OPANA ER is contraindicated in any situation where opioids are contraindicated such as: patients with respiratory depression (in the absence of resuscitative equipment or in unmonitored settings), and in patients with acute or severe bronchial asthma or hypercarbia.
OPANA ER, like all opioids, is contraindicated in any patient who has or is suspected of having paralytic ileus.
OPANA ER is contraindicated in patients with moderate and severe hepatic impairment (see CLINICAL PHARMACOLOGY, PRECAUTIONS and DOSAGE AND ADMINISTRATION).
WARNINGS
OPANA ER TABLETS are to be swallowed whole, and are not to be broken, chewed, crushed or dissolved. Taking broken, chewed, crushed or dissolved OPANA ER TABLETS could lead to the rapid release and absorption of a potentially fatal dose of oxymorphone.
Patients must not consume alcoholic beverages, or prescription or non-prescription medications containing alcohol, while on OPANA ER therapy. The co-ingestion of alcohol with OPANA ER may result in increased plasma levels and a potentially fatal overdose of oxymorphone.
Misuse, Abuse and Diversion of Opioids
OPANA ER contains oxymorphone, an opioid agonist similar to morphine, and is a Schedule II controlled substance. Opioid agonists have the potential for being abused and are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
Oxymorphone can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing OPANA ER in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion.
OPANA ER tablets may be abused by crushing, chewing, snorting or injecting the product. These practices will result in the uncontrolled delivery of the opioid and pose a significant risk to the abuser that could result in overdose and death (see WARNINGS and WARNINGS: Drug Abuse and Addiction).
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain.
Healthcare professionals should contact their State Professional Licensing Board, or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Interactions with Alcohol and Drugs of Abuse
Oxymorphone may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression because respiratory depression, hypotension, and profound sedation or coma may result. An in vivo study examined the effect of alcohol (40%, 20%, 4% and 0%) on the bioavailability of a single dose of 40 mg of OPANA ER in healthy, fasted volunteers. The results showed that the oxymorphone mean AUC was 13% higher (not statistically significant) after co-administration of 240 mL of 40% alcohol. The AUC was essentially unaffected in subjects following the co-administration of OPANA ER and ethanol (240 mL of 20% or 4% ethanol).
There was a highly variable effect on Cmax with concomitant administration of alcohol and OPANA ER. The change in Cmax ranged from a decrease of 50% to an increase of 270% across all conditions studied. Following concomitant administration of 240 mL of 40% ethanol the Cmax increased on average by 70%, and up to 270% in individual subjects. Following the concomitant administration of 240mL of 20% ethanol the Cmax increased on average by 31% and up to 260% in individual subjects. Following the concomitant administration of 240 mL of 4 % ethanol, the Cmax increased by 7% on average and as much as 110% for individual subjects.
Drug Abuse and Addiction
Controlled Substance
OPANA ER contains oxymorphone, an opioid with an abuse liability similar to morphine and other opioid agonists and is a Schedule II controlled substance. OPANA ER and other opioids used in analgesia, can be abused and are subject to criminal diversion (see WARNINGS: Misuse, Abuse and Diversion of Opioids).
Drug addiction is characterized by a preoccupation with the procurement, hoarding, and abuse of drugs for non-medicinal purposes. Drug addiction is treatable, utilizing a multi-disciplinary approach, but relapse is common.
"Drug seeking" behavior is very common to addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated claims of loss of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” (visiting multiple prescribers) to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction. Preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. OPANA ER, like other opioids, may be diverted for non-medical use. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Abuse of OPANA ER poses a risk of overdose and death. This risk is increased with concurrent abuse of OPANA ER with alcohol and other substances. In addition, parenteral drug abuse is commonly associated with transmission of infectious disease such as hepatitis and HIV.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal symptoms (see PRECAUTIONS: Usage in Pregnancy and PRECAUTIONS: Labor and Delivery).
Respiratory Depression
Respiratory depression is the chief hazard of OPANA ER. Respiratory depression is a particular potential problem in elderly or debilitated patients as well as in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation.
OPANA ER should be administered with extreme caution to patients with conditions accompanied by hypoxia, hypercapnia, or decreased respiratory reserve such as: asthma, chronic obstructive pulmonary disease or cor pulmonale, severe obesity, sleep apnea syndrome, myxedema, kyphoscoliosis, CNS depression or coma. In these patients, even usual therapeutic doses of oxymorphone may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea. Alternative non-opioid analgesics should be considered, and oxymorphone should be employed only under careful medical supervision at the lowest effective dose in such patients.
Interactions with Other Central Nervous System Depressants
Patients receiving other opioid analgesics, general anesthetics, phenothiazines or other tranquilizers, sedatives, hypnotics, or other CNS depressants (including alcohol) concomitantly with oxymorphone may experience respiratory depression, hypotension, profound sedation, or coma (see PRECAUTIONS: Drug-Drug Interactions).
Head Injury and Increased Intracranial Pressure
In the presence of head injury, intracranial lesions or a preexisting increase in intracranial pressure, the possible respiratory depressant effects of opioid analgesics and their potential to elevate cerebrospinal fluid pressure (resulting from vasodilation following CO2 retention) may be markedly exaggerated. Furthermore, opioid analgesics can produce effects on pupillary response and consciousness, which may obscure neurologic signs of further increases in intracranial pressure in patients with head injuries.
Hypotensive Effect
OPANA ER, like all opioid analgesics, may cause severe hypotension in an individual whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs such as phenothiazines or other agents which compromise vasomotor tone. OPANA ER, like all opioid analgesics, should be administered with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
Hepatic Impairment
A study of OPANA ER in patients with hepatic disease indicated greater plasma concentrations than those with normal hepatic function (see CLINICAL PHARMACOLOGY). OPANA ER should be used with caution in patients with mild impairment. These patients should be started with the lowest dose and titrated slowly while carefully monitoring for side effects. OPANA ER is contraindicated for patients with moderate and severe hepatic impairment (see CONTRAINDICATIONS, WARNINGS, and DOSAGE AND ADMINISTRATION).
PRECAUTIONS
General
Opioid analgesics should be used with caution especially when combined with other drugs, and should be reserved for cases where the benefits of opioid analgesia outweigh the known potential risks of respiratory depression, altered mental state and postural hypotension. OPANA ER should be used with caution in elderly and debilitated patients and in patients who are known to be sensitive to central nervous system depressants, such as those with cardiovascular, pulmonary, renal, or hepatic disease.
OPANA ER should be used with caution in the following conditions: acute alcoholism; adrenocortical insufficiency (e.g., Addison's disease); CNS depression or coma; delirium tremens; kyphoscoliosis associated with respiratory depression; myxedema or hypothyroidism; prostatic hypertrophy or urethral stricture; severe impairment of pulmonary or renal function; moderate impairment of hepatic function; and toxic psychosis.
The administration of oxymorphone may obscure the diagnosis or clinical course in patients with acute abdominal conditions. Oxymorphone may aggravate convulsions in patients with convulsive disorders, and all opioids may induce or aggravate seizures in some clinical settings.
OPANA ER is intended for use in patients who require more than several days continuous treatment with an opioid analgesic.
Ambulatory Surgery and Post-Operative Use
OPANA ER is not indicated for pre-emptive analgesia (administration pre-operatively for the management of post-operative pain).
OPANA ER is not indicated for pain in the immediate post-operative period (12-24 hours following surgery) for patients not previously taking opioids because of the risk of oversedation and respiratory depression requiring reversal with opioid antagonists.
OPANA ER is not indicated for pain in the post-operative period if the pain is mild or not expected to persist for an extended period of time.
OPANA ER is only indicated for postoperative use in the patient if the patient is already receiving the drug prior to surgery or if the postoperative pain is expected to be moderate to severe and persist for an extended period of time. Physicians should individualize treatment, moving from parenteral to oral analgesics as appropriate (see American Pain Society guidelines).
Patients who are already receiving OPANA ER as part of ongoing analgesic therapy may be safely continued on the drug if appropriate dosage adjustments are made considering the procedure, other drugs given, and the temporary changes in physiology caused by the surgical intervention (see DOSAGE AND ADMINISTRATION).
OPANA ER, like other opioids, decreases bowel motility. Ileus is a common post-operative complication, especially after intra-abdominal surgery with opioid analgesia. Caution should be taken to monitor for decreased bowel motility in post-operative patients receiving opioids. Standard supportive therapy should be implemented.
Use in Pancreatic/Biliary Tract Disease
OPANA ER, like other opioids, may cause spasm of the sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis.
Physical Dependence and Tolerance
Physical dependence is the occurrence of withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an opioid antagonist or mixed opioid agonist/antagonist agent. Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). The development of physical dependence and tolerance is not unusual during chronic opioid therapy.
If OPANA ER is abruptly discontinued in a physically-dependent patient, an abstinence syndrome may occur. Some or all of the following can characterize this syndrome: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
In general, OPANA ER should not be abruptly discontinued. However, OPANA ER, like other opioids, can be safely discontinued without the development of withdrawal symptoms by slowly tapering the daily dose (see DOSAGE AND ADMINISTRATION: Cessation of Therapy).
Information for Patients/Caregivers
- Patients should be advised that OPANA ER contains oxymorphone, a
morphine-like pain reliever, and should be taken only as directed.
- Patients should be advised that OPANA ER is designed to work properly only
if swallowed whole. The extended-release tablets may release all their contents
at once if broken, chewed or crushed, resulting in a risk of fatal overdose of
oxymorphone.
- Patients must not consume alcoholic beverages, or prescription or
non-prescription medications containing alcohol, while on OPANA ER therapy. The
co-ingestion of alcohol with OPANA ER may result in increased plasma levels and
a potentially fatal overdose of oxymorphone.
- Appropriate pain management requires changes in the dose to maintain best
pain control. Patients should be advised of the need to contact their physician
if pain control is inadequate, but not to change the dose of OPANA ER without
consulting their physician.
- Patients should be advised to report episodes of breakthrough pain and
adverse experiences occurring during therapy to their doctor. Individualization
of dosage is essential to make optimal use of this medication.
- Patients should be cautioned that OPANA ER may cause drowsiness, dizziness,
or lightheadedness, and may impair mental and/or physical abilities required for
the performance of potentially hazardous tasks, such as driving a car, operating
machinery, etc.
- Patients should not combine OPANA ER with alcohol or other central nervous
system depressants (sleep aids, tranquilizers) except by the orders of the
prescribing physician, because additive effects may occur, resulting in serious
injury or death.
- Patients taking OPANA ER should be advised of the potential for severe
constipation. Appropriate laxatives and/or stool softeners and other therapeutic
approaches may be considered for use with the initiation of OPANA ER
therapy.
- Patients should be advised not to adjust the dose of OPANA ER without
consulting the prescribing professional.
- Patients should be advised that OPANA ER is a potential drug of abuse. They
should protect it from theft, and it should never be given to anyone other than
the individual for whom it was prescribed.
- Women of childbearing potential who become, or are planning to become
pregnant should be advised to consult their physician regarding the effects of
opioid analgesics and other drug use during pregnancy on themselves and their
unborn child.
- If patients have been receiving treatment with OPANA ER for more than a few
days to weeks and cessation of therapy is indicated, they should be counseled on
the importance of safely tapering the dose and that abruptly discontinuing the
medication could precipitate withdrawal symptoms. The physician should determine
a dose schedule to accomplish a gradual discontinuation of the
medication.
- As with any potent opioid, misuse of OPANA ER may result in serious adverse events. Patients should be instructed to keep OPANA ER in a secure place out of the reach of children and pets. Accidental consumption especially in children can result in overdose or death. When OPANA ER is no longer needed, the unused tablets should be destroyed by flushing down the toilet.
Use in Drug and Alcohol Addiction
OPANA ER is not approved for use in detoxification or maintenance treatment of opioid addiction. However, the history of an addictive disorder does not necessarily preclude the use of this medication for the treatment of chronic pain. These patients will require intensive monitoring for signs of misuse, abuse, or addiction.
Drug-Drug Interactions
Oxymorphone is highly metabolized principally in the liver and undergoes reduction or conjugation with glucuronic acid to form both active and inactive metabolites (see Pharmacokinetics: Metabolism).
Use with CNS Depressants
The concomitant use of other CNS depressants including sedatives, hypnotics, tranquilizers, general anesthetics, phenothiazines, other opioids, and alcohol may produce additive CNS depressant effects. OPANA ER, like all opioid analgesics, should be started at 1/3 to 1/2 of the usual dose in patients who are concurrently receiving other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers, and alcohol because respiratory depression, hypotension, and profound sedation or coma may result, and titrated slowly as necessary for adequate pain relief.
Additive effects resulting in respiratory depression, hypotension, profound sedation or coma may result if these drugs are taken in combination with the usual doses of OPANA ER. No specific interaction between oxymorphone and monoamine oxidase inhibitors has been observed, but caution in the use of any opioid in patients taking this class of drugs is appropriate.
When combined therapy with any of the above medications is contemplated, the dose of one or both agents should be reduced (see WARNINGS and DOSAGE AND ADMINISTRATION).
Interactions with Mixed Agonist/Antagonist Opioid Analgesics
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol, or buprenorphine) should not be administered to patients who have received or are receiving a course of therapy with a pure opioid agonist analgesic, such as OPANA ER. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of OPANA ER and/or may precipitate withdrawal symptoms.
Other
Anticholinergics or other medications with anticholinergic activity when used concurrently with opioid analgesics may result in increased risk of urinary retention and/or severe constipation, which may lead to paralytic ileus.
In addition, CNS side effects have been reported (confusion, disorientation, respiratory depression, apnea, seizures) following coadministration of cimetidine with opioid analgesics; no clear-cut cause and effect relationship was established.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Long-term studies have been completed to evaluate the carcinogenic potential of oxymorphone in both Sprague-Dawley rats and CD-1 mice. Oxymorphone HCl was administered to Sprague-Dawley rats (2.5, 5, and 10 mg/kg/day in males and 5, 10, and 25 mg/kg/day in females) for 2 years by oral gavage. The systemic drug exposure (AUC ng•h/mL) at the 10 mg/kg/day in male rats was 0.34-fold and at the 25 mg/kg/day dose in female rats was 1.5-fold the human exposure at a dose of 260 mg/day. No evidence of carcinogenic potential was observed in rats. Oxymorphone HCl was administered to CD-1 mice (10, 25, 75 and 150 mg/kg/day) for 2 years by oral gavage. The systemic drug exposure (AUC ng•h/mL) at the 150 mg/kg/day dose in mice was 14.5-fold (in males) and 17.3-fold (in females) times the human exposure at a dose of 260 mg/day. No evidence of carcinogenic potential was observed in mice.
Mutagenesis: Oxymorphone hydrochloride was not mutagenic when tested in the in vitro bacterial reverse mutation assay (Ames test) at concentrations of ≤5270 µg/plate, or in an in vitro mammalian cell chromosome aberration assay performed with human peripheral blood lymphocytes at concentrations ≤5000 µg/ml with or without metabolic activation. Oxymorphone hydrochloride tested positive in both the rat and mouse in vivo micronucleus assays. An increase in micronucleated polychromatic erythrocytes occurred in mice given doses ≥250 mg/kg and in rats given doses of 20 and 40 mg/kg. A subsequent study demonstrated that oxymorphone hydrochloride was not aneugenic in mice following administration of up to 500 mg/kg. Additional studies indicate that the increased incidence of micronucleated polychromatic erythrocytes in rats may be secondary to increased body temperature following oxymorphone administration. Doses associated with increased micronucleated polychromatic erythrocytes also produce a marked, rapid increase in body temperature. Pretreatment of animals with sodium salicylate minimized the increase in body temperature and prevented the increase in micronucleated polychromatic erythrocytes after administration of 40 mg/kg oxymorphone.
Impairment of fertility: Oxymorphone hydrochloride did not affect reproductive function or sperm parameters in male rats at any dose tested (≤50 mg/kg/day). In female rats, an increase in the length of the estrus cycle and decrease in the mean number of viable embryos, implantation sites and corpora lutea were observed at doses of oxymorphone ≥10 mg/kg/day. The dose of oxymorphone associated with reproductive findings in female rats is 1.2-fold the human dose of 40 mg every 12 hours based on a body surface area. The dose of oxymorphone that produced no adverse effects on reproductive findings in female rats is 0.6-fold the human dose of 40 mg every 12 hours on a body surface area basis.
Pregnancy
The safety of using oxymorphone in pregnancy has not been established with regard to possible adverse effects on fetal development. The use of OPANA ER in pregnancy, in nursing mothers, or in women of child-bearing potential requires that the possible benefits of the drug be weighed against the possible hazards to the mother and the child (see PRECAUTIONS).
Teratogenic Effects
Pregnancy Category C
Oxymorphone hydrochloride administration did not cause malformations at any doses evaluated during developmental toxicity studies in rats (≤25 mg/kg/day) or rabbits (≤50 mg/kg/day). These doses are ~3-fold and ~12-fold the human dose of 40 mg every 12 hours, based on body surface area. There were no developmental effects in rats treated with 5 mg/kg/day or rabbits treated with 25 mg/kg/day. Fetal weights were reduced in rats and rabbits given doses of ≥10 mg/kg/day and 50 mg/kg/day, respectively. These doses are ~1.2-fold and ~6-fold the human dose of 40 mg every 12 hours based on body surface area, respectively. There were no effects of oxymorphone hydrochloride on intrauterine survival in rats at doses ≤25 mg/kg/day, or rabbits at ≤50 mg/kg/day in these studies (see Non-teratogenic Effects, below). In a study that was conducted prior to the establishment of Good Laboratory Practices (GLP) and not according to current recommended methodology, a single subcutaneous injection of oxymorphone hydrochloride on gestation day 8 was reported to produce malformations in offspring of hamsters that received 15.5-fold the human dose of 40 mg every 12 hours based on body surface area. This dose also produced 83% maternal lethality.
There are no adequate and well-controlled studies in pregnant women. OPANA ER should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Non-teratogenic Effects
Oxymorphone hydrochloride administration to female rats during gestation in a pre- and postnatal developmental toxicity study reduced mean litter size (18%) at a dose of 25 mg/kg/day, attributed to an increased incidence of stillborn pups. An increase in neonatal death occurred at ≥5 mg/kg/day. Post-natal survival of the pups was reduced throughout weaning following treatment of the dams with 25 mg/kg/day. Low pup birth weight and decreased postnatal weight gain occurred in pups born to oxymorphone-treated female rats given a dose of 25 mg/kg/day. This dose is ~3-fold higher than the human dose of 40 mg every 12 hours on a body surface area basis.
Prolonged use of opioid analgesics during pregnancy may cause fetal-neonatal physical dependence. Neonatal withdrawal may occur. Symptoms usually appear during the first days of life and may include convulsions, irritability, excessive crying, tremors, hyperactive reflexes, fever, vomiting, diarrhea, sneezing, yawning, and increased respiratory rate.
Labor and Delivery
Opioids cross the placenta and may produce respiratory depression and psycho-physiologic effects in neonates. OPANA ER is not recommended for use in women during and immediately prior to labor, when use of shorter acting analgesics or other analgesic techniques are more appropriate. Occasionally, opioid analgesics may prolong labor through actions which temporarily reduce the strength, duration and frequency of uterine contractions. However this effect is not consistent and may be offset by an increased rate of cervical dilatation, which tends to shorten labor. Neonates whose mothers received opioid analgesics during labor should be observed closely for signs of respiratory depression. A specific opioid antagonist, such as naloxone or nalmefene, should be available for reversal of opioid-induced respiratory depression in the neonate.
Nursing Mothers
It is not known whether oxymorphone is excreted in human milk. Because many drugs, including some opioids, are excreted in human milk, caution should be exercised when OPANA ER is administered to a nursing woman. Ordinarily, nursing should not be undertaken while a patient is receiving oxymorphone because of the possibility of sedation and/or respiratory depression in the infant.
Pediatric Use
Safety and effectiveness of OPANA ER in pediatric patients below the age of 18 years have not been established.
Geriatric Use
OPANA ER should be used with caution in elderly patients. The plasma levels of oxymorphone are about 40% higher in elderly (≥65 years of age) than in younger subjects (see CLINICAL PHARMACOLOGY). Elderly patients should initially receive smaller starting doses of oxymorphone and dose titration should proceed cautiously.
Of the total number of subjects in clinical studies of OPANA ER, 27 percent were 65 and over, while 9 percent were 75 and over. No overall differences in effectiveness were observed between these subjects and younger subjects. There were several adverse events that were more frequently observed in subjects 65 and over compared to younger subjects. These adverse events included dizziness, somnolence, confusion, and nausea.
Hepatic Impairment
A study of OPANA ER in patients with hepatic disease indicated greater plasma concentrations than those with normal hepatic function (see CLINICAL PHARMACOLOGY). OPANA ER should be used with caution in patients with mild impairment. These patients should be started with the lowest dose and titrated slowly while carefully monitoring for side effects. OPANA ER is contraindicated for patients with moderate and severe hepatic impairment (see CONTRAINDICATIONS, WARNINGS, and DOSAGE AND ADMINISTRATION).
Renal Impairment
In a study of OPANA ER, patients with moderate to severe renal impairment were shown to have an increase in bioavailability ranging from 57-65% (see CLINICAL PHARMACOLOGY). These patients should be started cautiously with lower doses of OPANA ER and titrated slowly while carefully monitored for side effects (see DOSAGE AND ADMINISTRATION).
Gender Differences
When normalized for body weight, gender differences were not observed (see CLINICAL PHARMACOLOGY). In clinical studies, the overall incidence rates for one or more adverse events were slightly higher among females than males for both OPANA ER subjects and placebo subjects.
ADVERSE REACTIONS
Tables 2 and 3 list the most frequently occurring adverse reactions (in at least 5% of patients) from the placebo-controlled trials in patients with low back pain.
|
| Open-Label Titration Period | Double-Blind Treatment Period | |
|
| OPANA ER | OPANA ER | Placebo |
| Preferred Term | (N = 325) | (N = 105) | (N = 100) |
|
|
|
|
|
| Constipation | 26.2% | 6.7% | 1.0% |
| Somnolence | 19.1% | 1.9% | 0% |
| Nausea | 18.2% | 11.4% | 9.0% |
| Dizziness | 11.1% | 4.8% | 3.0% |
| Headache | 10.5% | 3.8% | 2.0% |
| Pruritus | 6.8% | 2.9% | 1.0% |
|
| Open-Label Titration Period | Double-Blind Treatment Period | |
|
| OPANA ER | OPANA ER | Placebo |
| Preferred Term | (N = 250) | (N = 70) | (N = 72) |
|
|
|
|
|
| Nausea | 19.6% | 2.9% | 1.4% |
| Constipation | 11.6% | 5.7% | 1.4% |
| Headache | 11.6% | 2.9% | 0% |
| Somnolence | 11.2% | 2.9% | 0% |
| Vomiting | 8.8% | 0% | 1.4% |
| Pruritus | 7.6% | 0% | 0% |
| Dizziness | 6.4% | 0% | 0% |
The following table lists adverse reactions that were reported in at least 2% of patients in placebo-controlled trials (N=5)
| MedDRA Preferred Term | OPANA ER (N=1259) | Placebo (N=461) |
| Nausea | 33.1% | 13.2% |
| Constipation | 27.6% | 13.2% |
| Dizziness (Exc Vertigo) | 17.8% | 7.6% |
| Somnolence | 17.2% | 2.2% |
| Vomiting | 15.6% | 4.1% |
| Pruritus | 15.2% | 7.6% |
| Headache | 12.2% | 5.6% |
| Sweating increased | 8.6% | 8.7% |
| Dry mouth | 6.4% | 0.7% |
| Sedation | 5.9% | 7.6% |
| Diarrhea | 4.3% | 5.6% |
| Insomnia | 4.0% | 2.0% |
| Fatigue | 3.9% | 1.3% |
| Appetite decreased | 2.9% | 0.4% |
| Abdominal pain | 2.5% | 1.5% |
Adverse Reactions Reported in All Clinical Trials
A total of 2011 patients were treated with OPANA ER in the Phase 2/3 controlled and open-label clinical trials. The clinical trials consisted of patients with moderate to severe chronic pain and post surgical pain.
The adverse reactions are presented in the following manner: most common, common, and less common adverse reactions.
The most common adverse drug reactions (≥10%) reported at least once by patients treated with OPANA ER in the clinical trials were nausea, constipation, dizziness (exc. vertigo), vomiting, pruritus, somnolence, headache, sweating increased, and sedation.
The common (≥1% - less than 10%) adverse drug reactions reported at least once by patients treated with OPANA ER in the clinical trials organized by MedDRA’s (Medical Dictionary for Regulatory Activities) System Organ Class were:
Eye disorders: vision blurred
Gastrointestinal disorders: diarrhea, abdominal pain, dyspepsia
General disorders and administration site conditions: dry mouth, appetite decreased, fatigue, lethargy, weakness, pyrexia, dehydration, weight decreased, edema
Nervous system disorders: insomnia
Psychiatricdisorders: anxiety, confusion, disorientation, restlessness, nervousness, depression
Respiratory, thoracic and mediastinal disorders: dyspnea
Vascular disorders: flushing and hypertension
Other less common adverse reactions known with opioid treatment that were seen less than 1% in the OPANA ER trials include the following in alphabetical order.
Abdominal distention, agitation, allergic reactions, bradycardia, central nervous system depression, clamminess, depressed level of consciousness, dermatitis, difficult micturition, dysphoria, euphoric mood, feeling jittery, hallucination, hot flashes, hypersensitivity, hypotension, hypoxia, ileus, mental impairment, mental status changes, miosis, oxygen saturation decreased, palpitation, postural hypotension, respiratory depression, respiratory distress, respiratory rate decreased, syncope, tachycardia, urinary retention, urticaria, and visual disturbances.
OVERDOSAGE
Signs and Symptoms
Acute overdosage with OPANA ER is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils and sometimes bradycardia and hypotension. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur.
OPANA ER may cause miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations (see CLINICAL PHARMACOLOGY: Central Nervous System).
Treatment
In the treatment of OPANA ER overdosage, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation. Elimination or evacuation of gastric contents may be necessary in order to eliminate unabsorbed drug. Before attempting treatment by gastric emptying or activated charcoal, care should be taken to secure the airway.
The opioid antagonist naloxone hydrochloride is a specific antidote against respiratory depression, which may result from overdosage or unusual sensitivity to opioids including OPANA ER. Therefore, an appropriate dose of naloxone hydrochloride should be administered (usual initial adult dose 0.4 mg-2 mg) preferably by the intravenous route and simultaneously with efforts at respiratory resuscitation. Nalmefene is an alternative pure opioid antagonist, which may be administered as a specific antidote to respiratory depression resulting from opioid overdose. Since the duration of action of OPANA ER may exceed that of the antagonist, the patient should be kept under continued surveillance and repeated doses of the antagonist should be administered according to the antagonist labeling as needed to maintain adequate respiration.
In patients receiving OPANA ER, opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to OPANA ER overdose. They should be administered cautiously to persons who are known, or suspected to be, physically dependent on any opioid agonist including OPANA ER. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome. In an individual physically dependent on opioids, administration of the usual dose of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. If respiratory depression is associated with muscular rigidity, administration of a neuromuscular blocking agent may be necessary to facilitate assisted or controlled ventilation. Muscular rigidity may also respond to opioid antagonist therapy
DOSAGE AND ADMINISTRATION
OPANA ER Tablets are to be swallowed whole and are not to be broken, chewed, dissolved, or crushed. Taking broken, chewed, dissolved, or crushed OPANA ER Tablets leads to rapid release and absorption of a potentially fatal dose of oxymorphone.
Patients must not consume alcoholic beverages, or prescription or non-prescription medications containing alcohol, while on OPANA ER therapy. The co-ingestion of alcohol with OPANA ER may result in increased plasma levels and a potentially fatal overdose of oxymorphone.
OPANA ER is an opioid agonist and a Schedule II controlled substance with an abuse liability similar to morphine and other opioids.
OPANA ER, like morphine and other opioids used in analgesia, can be abused and is subject to criminal diversion.
OPANA ER tablets are to be swallowed whole, and are not to be broken, chewed, crushed or dissolved. Taking broken, chewed, crushed or dissolved OPANA ER tablets leads to the rapid release and absorption of a potentially fatal dose of oxymorphone.
While symmetric (same dose AM and PM), around-the-clock, every 12 hours dosing is appropriate for the majority of patients, some patients may benefit from asymmetric (different dose given in AM than in PM) dosing, tailored to their pain pattern. It is usually appropriate to treat a patient with only one extended-release opioid for around-the-clock therapy.
Selection of patients for treatment with OPANA ER should be governed by the same principles that apply to the use of other extended-release opioid analgesics (see INDICATIONS AND USAGE). As with any opioid drug product, it is necessary to adjust the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment experience. Physicians should individualize treatment in every case (see DOSAGE AND ADMINISTRATION), using non-opioid analgesics, prn opioids and/or combination products, and chronic opioid therapy in a progressiveplan of pain management such as outlined by the World Health Organization, the American Pain Society and the Federation of State Medical Boards Model Guidelines. Healthcare professionals should follow appropriate pain management principles of careful assessment and ongoing monitoring (see BOXED WARNING).
In the selection of the initial dose of OPANA ER, attention should be given to the following:
- The total daily dose, potency and specific characteristics of the opioid the patient has been taking previously;
- The relative potency estimate used to calculate the equivalent oxymorphone dose needed;
- The patient’s degree of opioid tolerance;
- The age, general condition, and medical status of the patient;
- Concurrent non-opioid analgesic and other medications;
- The type and severity of the patient's pain;
- The balance between pain control and adverse experiences;
- Risk factors for abuse, addiction or diversion, including a prior history of abuse, addiction or diversion.
The following dosing recommendations, therefore, can only be considered as suggested approaches to what is actually a series of clinical decisions over time in the management of the pain of each individual patient.
OPANA ER should be administered on an empty stomach, at least one hour prior to or two hours after eating.
Initiation of Therapy
Opioid-Naïve Patients
It is suggested that patients who are not opioid-experienced being initiated on chronic around-the-clock opioid therapy be started with OPANA ER 5 mg every 12 hours. Thereafter, it is recommended that the dose be individually titrated, preferably at increments of 5-10 mg every 12 hours every 3-7 days, to a level that provides adequate analgesia and minimizes side effects under the close supervision of the prescribing physician (see CLINICAL TRIALS: 12-Week Study in Opioid-Naïve Patients with Low Back Pain).
Opioid-Experienced PatientsConversion from OPANA to OPANA ER
Patients receiving OPANA may be converted to OPANA ER by administering half the patient's total daily oral OPANA dose as OPANA ER, every 12 hours. For example, a patient receiving 40 mg/day OPANA may require 20 mg OPANA ER every 12 hours.
Conversion from Parenteral Oxymorphone to OPANA ER
Given the absolute oral bioavailability of approximately 10%, patients receiving parenteral oxymorphone may be converted to OPANA ER by administering 10 times the patient's total daily parenteral oxymorphone dose as OPANA ER in two equally divided doses (e.g., IV dose x 10/2). For example, approximately 20 mg of OPANA ER, every 12 hours, may be required to provide pain relief equivalent to a total daily dose of 4 mg of parenteral oxymorphone. Due to patient variability with regards to opioid analgesic response, upon conversion patients should be closely monitored to ensure adequate analgesia and to minimize side effects.
Conversion from Other Oral Opioids to OPANA ER
For conversion from other opioids to OPANA ER, physicians and other healthcare professionals are advised to refer to published relative potency information, keeping in mind that conversion ratios are only approximate. In general, it is safest to start the OPANA ER therapy by administering half of the calculated total daily dose of OPANA ER (see conversion ratio table below) in 2 divided doses, every 12 hours. The initial dose of OPANA ER can be gradually adjusted until adequate pain relief and acceptable side effects have been achieved. The following table provides approximate equivalent doses, which may be used as a guideline for conversion. The conversion ratios and approximate equivalent doses in this conversion table are only to be used for the conversion from current opioid therapy to OPANA ER. In a Phase 3 clinical trial with an open-label titration period, patients were converted from their current opioid to OPANA ER using the following table as a guide. In general, patients were able to successfully titrate to a stabilized dose of OPANA ER within 4 weeks (see Clinical TRIALS: 12-Week Study in Opioid-Experienced Patients with Low Back Pain). There is substantial patient variation in the relative potency of different opioid drugs and formulations.
CONVERSION RATIOS TO OPANA ERApproximate Equivalent Dose
| Opioid | Oral | Oral Conversion Ratio a |
| Oxymorphone | 10 mg | 1 |
| Hydrocodone | 20 mg | 0.5 |
| Oxycodone | 20 mg | 0.5 |
| Methadone | 20 mg | 0.5 |
| Morphine | 30 mg | 0.333 |
the dose by the conversion ratio to calculate the approximate oral oxymorphone equivalent.
- The conversion ratios and approximate equivalent doses in this conversion table are only to be used for the conversion from current opioid therapy to Opana ER.
- Sum the total daily dose for the opioid and multiply by the conversion ratio to calculate the oxymorphone total daily dose.
- For patients on a regimen of mixed opioids, calculate the approximate oral oxymorphone dose for each opioid and sum the totals to estimate the total daily oxymorphone dose.
- The dose of OPANA ER can be gradually adjusted, preferably at increments if 10 mg every 12 hours every 3-7 days, until adequate pain relief and acceptable side effects have achieved (see individualization of Dose).
Individualization of Dose
Once therapy is initiated, pain relief and other opioid effects should be frequently assessed. In clinical practice, titration of the total daily OPANA ER dose should be based upon the amount of supplemental opioid utilization, severity of the patient’s pain, and the patient’s ability to tolerate the opioid. Patients should be titrated to generally mild or no pain with the regular use of no more than two doses of supplemental analgesia, i.e. “rescue,” per 24 hours.
If signs of excessive opioid-related adverse experiences are observed, the next dose may be reduced. If this adjustment leads to inadequate analgesia, a supplemental dose of OPANA, another immediate-release opioid, or a non-opioid analgesic may be administered. Dose adjustments should be made to obtain an appropriate balance between pain relief and opioid-related adverse experiences. If significant adverse events occur before the therapeutic goal of mild or no pain is achieved, the events should be treated aggressively. Once adverse events are under control, upward titration should continue to an acceptable level of pain control.
During periods of changing analgesic requirements, including initial titration, frequent contact is recommended between physician, other members of the healthcare team, the patient and the caregiver/family. Patients and caregivers/family members should be advised of the potential side effects.
Patients with Hepatic Impairment
Patients with mild hepatic impairment should be started with the lowest dose and titrated slowly while carefully monitoring side effects. OPANA ER is contraindicated in patients with moderate and severe hepatic dysfunction (see CLINICAL PHARMACOLOGY, CONTRAINDICATIONS and PRECAUTIONS).
Patients with Renal Impairment
There are 57% and 65% increases in oxymorphone bioavailability in patients with moderate and severe renal impairment, respectively (see CLINICAL PHARMACOLOGY and PRECAUTIONS). Accordingly, in patients with creatinine clearance rate less than 50 mL/min, OPANA ER should be started with the lowest dose and titrated slowly while carefully monitoring side effects.
Use with CNS Depressants
OPANA ER, like all opioid analgesics, should be started at 1/3 to 1/2 of the usual dose in patients who are concurrently receiving other central nervous system depressants including sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers, and alcohol because respiratory depression, hypotension, and profound sedation or coma may result. No specific interaction between oxymorphone and monoamine oxidase inhibitors has been observed, but caution in the use of any opioid in patients taking this class of drugs is appropriate (see PRECAUTIONS: General and PRECAUTIONS: Drug-Drug Interactions).
Geriatrics
The steady-state plasma concentrations of oxymorphone are approximately 40% higher in elderly subjects than in young subjects (see CLINICAL PHARMACOLOGY and PRECAUTIONS). In general, caution should be exercised in the selection of the starting dose of OPANA ER for an elderly patient usually starting at the low end of the dosing range and slowly titrating to adequate analgesia.
Maintenance of Therapy and Supplemental Analgesia
The intent of the titration period is to establish a patient-specific every 12 hours dose that will maintain adequate analgesia with acceptable side effects for as long as pain relief is necessary. During titration and before a stable dose is achieved, OPANA or other immediate-release medications can be used as supplemental analgesia between dosings. Should pain recur, the dose can be incrementally increased to re-establish pain control. The method of therapy adjustment outlined above should be employed to re-establish pain control.
During chronic therapy with OPANA ER, the continued need for around-the-clock opioid therapy should be reassessed periodically.
Cessation of Therapy
When the patient no longer requires therapy with OPANA ER tablets, doses should be tapered gradually to prevent signs and symptoms of withdrawal in the physically dependent patient (see CLINICAL TRIALS: 12-Week Study in Opioid-Naïve Patients with Low Back Pain and CLINICAL TRIALS: 12-Week Study in Opioid-Experienced Patients with Low Back Pain).
SAFETY AND HANDLING
OPANA ER contains oxymorphone, which is a controlled substance. Oxymorphone is controlled under Schedule II of the Controlled Substances Act. Oxymorphone, like all opioids, is liable to diversion and misuse and should be handled accordingly. Patients and their families should be instructed to flush any OPANA ER tablets that are no longer needed.
OPANA ER may be targeted for theft and diversion. Healthcare professionals should contact their State Medical Board, State Board of Pharmacy or State Control Board for information on how to detect or prevent diversion of this product.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature].
Dispense in tight container as defined in the USP, with a child-resistant closure (as required).
HOW SUPPLIED
OPANA ER tablets are supplied as follows:
5 mg
Pink, octagon shape, film coated, convex
tablets with “E907” over “5” in black print on one side and plain on the
other.
Bottles of 100 with child-resistant
closure NDC 63481-907-70
Unit-Dose package
of 100 tablets (5 blister cards of 20
tablets, not child-resistant, for
hospital use only) NDC 63481-907-75
10 mg
Light orange, octagon shape, film coated,
convex tablets with “E674” over “10” in black print on one side and plain on the
other.
Bottles of 100 with child-resistant
closure NDC 63481-674-70
Unit-Dose package
of 100 tablets (5 blister cards of 20
tablets, not child-resistant, for
hospital use only) NDC 63481-674-75
20 mg
Light green, octagon shape, film coated,
convex tablets with “E617” over “20” in black print on one side and plain on the
other.
Bottles of 100 with child-resistant
closure NDC 63481-617-70
Unit-Dose package
of 100 tablets (5 blister cards of 20
tablets, not child-resistant, for
hospital use only) NDC 63481-617-75
40 mg
Yellow, octagon shape, film coated, convex
tablets with “E693” over “40” in black print on one side and plain on the
other.
Bottles of 100 with child-resistant
closure NDC 63481-693-70
Unit-Dose package
of 100 tablets (5 blister cards of 20
tablets, not child-resistant, for
hospital use only) NDC 63481-693-75
Rx Only
CAUTION
DEA Order Form
Required.
PATIENT INFORMATION
OPANA® ER
(Ō-pan-a)
(Oxymorphone Hydrochloride) Extended-Release Tablets
CII
Rx Only
OPANA ER Tablets, 5 mg
OPANA ER Tablets, 10 mg
OPANA ER Tablets, 20
mg
OPANA ER Tablets, 40 mg
| IMPORTANT: Keep OPANA ER in a safe place away from children. Accidental use by a child is a medical emergency and can result in death. If a child accidentally takes OPANA ER, get emergency help right away. |
Read the Patient Information that comes with OPANA ER before you start taking it and each time you get a new prescription. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. Share the important information in this leaflet with members of your household.
What Is the Most Important Information I Should Know About OPANA ER?
-
OPANA ER can cause trouble breathing (hypoventilation),
which can lead to death, if used differently than the way you were told
to use it by your healthcare provider (see “What are the possible side effects
of OPANA ER?”).
- Swallow OPANA ER tablets whole. Do not break, crush, dissolve, or chew OPANA ER tablets before swallowing. If a tablet is broken, crushed, dissolved, or chewed, the full 12 hour dose can be taken into your body all at once. This is very dangerous. You could die from an overdose of the medicine. Use OPANA ER exactly the way your healthcare provider prescribes. If you cannot swallow tablets whole, tell your healthcare provider. You may need a different medicine.
What is OPANA ER?
- OPANA ER is a prescription medicine that contains the opioid (narcotic pain
medicine) oxymorphone. OPANA ER is used to treat adults with constant pain
(around the clock) that is moderate to severe and is expected to last for an
extended period of time. OPANA ER is not for occasional (“as
needed”) use.
-
OPANA ER can cause physical dependence. Do not stop
taking OPANA ER all of a sudden if you have been taking it for more than a few
days. You could become sick with uncomfortable withdrawal symptoms because your
body has become use to the medicine. Talk to your healthcare provider about
slowly stopping OPANA ER to avoid getting sick with withdrawal symptoms.
Physical dependence is not the same as drug addiction. Your healthcare provider
can tell you more about the differences between physical dependence and drug
addiction.
- OPANA ER is a controlled substance (CII) because it contains a narcotic painkiller that can be a target for people who abuse prescription medicines or street drugs. Keep your tablets in a safe place to protect them from being stolen. Never give your tablets to anyone else, even if they have the same symptoms you have. Selling or giving away this medicine may harm others, even causing death, and is against the law.
Who Should Not Take OPANA ER?
Do
not take OPANA ER if:
- You had surgery within the past day (24 hours) and you were not taking OPANA
ER before your surgery.
- Your pain is mild or will go away in a few days.
- Your pain can be controlled by the occasional use of other pain
medicines.
- You are having an asthma attack or have severe asthma, trouble breathing, or
lung problems.
- You have liver problems.
- You are allergic to OPANA ER or anything in it. See the end of this leaflet for a complete list of ingredients in OPANA ER.
You have had severe allergic reactions to other narcotic pain medicines (such as morphine or codeine medicines). A severe allergic reaction includes a severe rash, hives, breathing problems, or dizziness.
OPANA ER is not for children under 18 years of age.
What Should I Tell My Healthcare Provider Before Starting
OPANA ER?
Tell your healthcare provider about all of
your medical problems, especially if you:
- have trouble breathing or lung problems
- have a head injury or brain problem
- have liver or kidney problems
- have adrenal gland problems, such as Addison’s disease
- have convulsions or seizures
- have thyroid problems
- have problems urinating or prostate problems
- have pancreas problems
- have a drinking problem or alcoholism
- have severe mental problems or hallucinations (see or hear things that are
not really there)
- have past or present drug abuse or drug addiction problems
-
are pregnant or plan to become pregnant. OPANA ER
may harm your unborn baby.
- are breastfeeding. OPANA ER may pass through your milk and may harm your baby. You should not breastfeed while taking OPANA ER.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may cause serious problems when taken with OPANA ER, especially if they cause sleepiness (like sleeping pills, anxiety medicines, antihistamines, or tranquilizers).
Do not take any new medicines while using OPANA ER until you have talked to your healthcare provider or pharmacist and they have told you it is safe.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist.
How Should I Take OPANA ER?
-
Follow your healthcare provider’s directions
exactly. Your healthcare provider may change your dose based on your
reactions to the medicine. Do not change your dose unless your healthcare
provider tells you to change it. Do not take OPANA ER more often than
prescribed.
-
Swallow OPANA ER tablets whole. Do not break, crush,
dissolve, or chew OPANA ER tablets before swallowing. If a tablet is broken,
crushed, dissolved, or chewed, the full 12 hour dose can be taken into your body
all at once. This is very dangerous. You could die from an overdose of the
medicine. If you cannot swallow tablets whole, tell your healthcare
provider. You may need a different medicine.
- Take OPANA ER every 12 hours or as instructed by your healthcare provider.
OPANA ER should be taken on an empty stomach, at least one hour before or two
hours after meals. Talk to your healthcare provider if you feel sick taking
OPANA ER on an empty stomach.
-
If you miss a dose, take it as soon as possible. If
it is almost time for your next dose, skip the missed dose and go back to your
regular dosing schedule. Do not take 2 doses at once unless
your healthcare provider tells you to. If you are not sure about your
dosing call your healthcare provider.
-
If you take too much OPANA ER or overdose, call your
local emergency number or poison control center right away.
-
Talk to your healthcare provider often about your pain.
Your healthcare provider can decide if you still need OPANA ER.
-
If you have side effects that bother you or if you continue
to have pain, call your healthcare provider.
- Stopping OPANA ER. If your healthcare provider decides you no longer need OPANA ER, ask how to slowly reduce the dose of your medicine so you don’t get uncomfortable (withdrawal) symptoms such as nausea, sweating, and pain. You should not stop taking OPANA ER all at once if you have been taking it for more than a few days without talking to your healthcare provider. OPANA ER can cause physical dependence. You can get sick with withdrawal symptoms if you stop OPANA ER all at once, because your body has become use to it.
After you stop taking OPANA ER, flush the unused tablets down the toilet. Safely dispose of OPANA ER out of the reach of children and pets.
What Should I Avoid While Taking OPANA ER?
-
Do not drive, operate heavy machinery, or participate in
any other possibly dangerous activities until you know how you react to
this medicine. OPANA ER can make you sleepy. Ask your healthcare provider to
tell you when it is okay to do these activities.
- Do not drink alcohol while using OPANA ER. It may increase the chance of having dangerous side effects including overdose and death.
What are the Possible Side Effects of OPANA ER?
OPANA ER can cause trouble breathing.
Call your healthcare provider or get medical help right away
if:
- your breathing slows down
- you have shallow breathing (little chest movement with breathing)
- you feel faint, dizzy, confused, or have any other unusual symptoms
These can be signs that you have taken too much OPANA ER (overdose) or the dose is too high for you, which can be dangerous and lead to death if not treated.
OPANA ER can cause your blood pressure to drop. This can make you feel dizzy if you get up too fast from sitting or lying down. Low blood pressure is also more likely to happen if you are taking other medicines that can also lower your blood pressure.
OPANA ER can cause physical dependence. Your body will get used to OPANA ER if you take it more than a few days. You can get sick with withdrawal symptoms if you stop taking OPANA ER all at once. You can avoid getting sick with withdrawal symptoms by stopping OPANA ER slowly. Your healthcare provider will tell you how to do this.
There is a chance of abuse or addiction with OPANA ER. Abuse or addiction is different than a physical dependence. If you have abused prescription medicines, street drugs or alcohol in the past, you may have a higher chance of developing abuse or addiction again while using OPANA ER. If you have more concerns, talk to your healthcare provider for more information about abuse and addiction.
The most common side effects of OPANA ER are nausea, constipation, dizziness, vomiting, itching, sleepiness, headache, increased sweating, and sedation. Some of these side effects may decrease with continued use. Talk to your healthcare provider if you continue to have these side effects.
These are not all the possible side effects of OPANA ER. For a complete list, ask your healthcare provider or pharmacist.
Constipation (decrease in the usual number of hard bowel movements) is a common side effect of opioid medicines, including OPANA ER. Talk to your healthcare provider or pharmacist about the use of laxatives (medicines to treat constipation) and stool softeners to prevent or treat constipation while taking OPANA ER.
How should I store OPANA ER?
- Store OPANA ER at room temperature between 59° to 86°F (15° to
30°C).
- Keep OPANA ER in a childproof container and store in a safe place to protect
it from being stolen.
- Keep OPANA ER out of the reach of children. Accidental overdose in children is an emergency and can result in death.
General Information about OPANA ER
- Do not use OPANA ER for conditions for which it was not prescribed.
- Do not give OPANA ER to other people, even if they have the same symptoms you have. It may harm them, even causing death, and it is against the law.
This leaflet summarizes the most important information about OPANA ER. If you would like more information, talk with your healthcare provider. Also, you can ask your pharmacist or healthcare provider for information about OPANA ER that is written for healthcare professionals.
For additional information, please go to www.Endo.com or www.opana.com
What are the ingredients in OPANA ER?
Active Ingredient:
oxymorphone hydrochloride
Inactive Ingredients:
hypromellose, iron oxide black, methylparaben, propylene glycol,
silicified microcrystalline cellulose, sodium stearyl fumarate, TIMERx®-N, titanium dioxide, and triacetin. The 5 mg, 10 mg and 20 mg
tablets also contain macrogol, and polysorbate 80. In addition, the 5 mg tablets
contain iron oxide red. The 10 mg tablets contain FD and C yellow No. 6. The 20
mg tablets contain FD and C blue No. 1, FD and C yellow No. 6, and D and C
yellow No. 10. The 40 mg tablets contain FD and C yellow No. 6, D and C yellow
No.10, and lactose monohydrate.
CAUTION: Federal law prohibits dispensing without prescription.
Manufactured for:
Endo Pharmaceuticals Inc.
Chadds Ford, Pennsylvania 19317
Manufactured by:
Novartis Consumer Health Inc.
Lincoln, NE 68517
TIMERx®-N is a registered Trademark of Penwest Pharmaceuticals Co., Danbury, Connecticut and is used herein pursuant to a license agreement between Penwest and Endo Pharmaceuticals.
Copyright © Endo Pharmaceuticals Inc. 2006
413442/June, 2006


