Principal for Oxygen Product
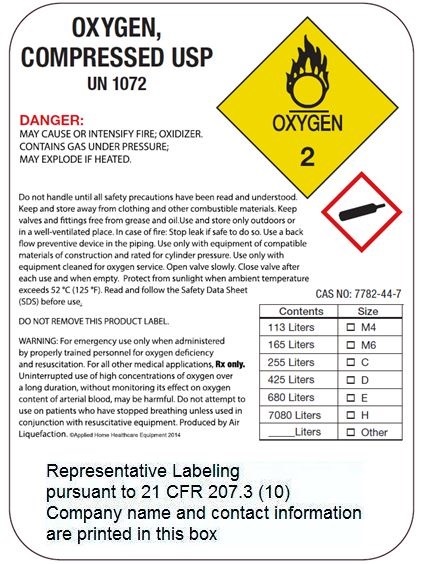
OXYGEN
COMPRESSED
USP
UN 1072
PRODUCED BY AIR LIQUIFICATION
WARNING: HIGH PRESSURE OXIDIZING GAS, VIGOROUSLY ACCELERATES COMBUSTION. NO SMOKING IN THE PRESENCE OF OXYGEN OR A FIRE MAY RESULT.
WARNING: For emergency use only when administered by properly trained personnel for oxygen deficiency and resuscitation. For all other medical applications, Rx only.
WARNING: Federal law prohibits dispensing without prescription. Uninterrupted use of high concentrations of oxygen over a long duration, without monitoring its effect on oxygen content of arterial blood, may be harmful. Use only with pressure reducing equipment and apparatus designed for oxygen. Do not attempt to use on patients who have stopped breathing, unless used in conjuction with resuscitative equipment.
WARNING: Keep oil and grease away. Use only with equipment cleaned for oxygen service and rated for cylinder pressure. Open valve slowly. Close valve after each use and when empty.
USE IN ACCORDANCE WITH MATERIAL SAFETY DATA SHEET.
DO NOT REMOVE THIS PRODUCT LABEL.
