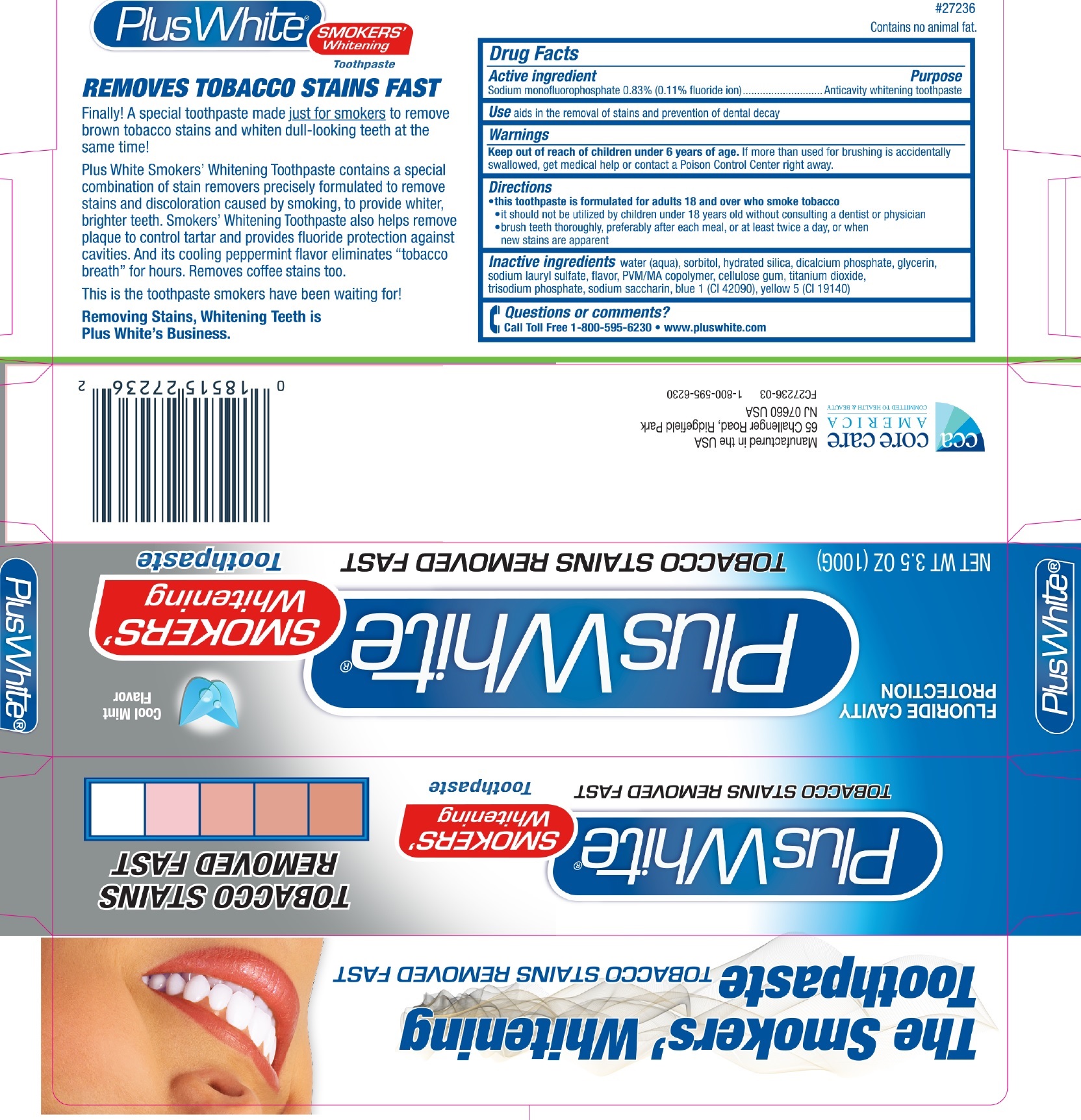
PLUS PLUS WHITE SMOKERS WHITENING- sodium monofluorophosphate paste
CCA Industries, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Sodium monofluorophosphate 0.83% (0.11% fluoride ion)
Purpose
Anticavity whitening toothpaste
Use
aids in the removal of stains and prevention of dental decay
Warnings
Keep out of reach of children under 6 years of age
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- this toothpaste is formulated for adults 18 and over who smoke tobacco
- it should not be utilized by children under 18 years old without consulting a dentist or physician
- brush teeth thoroughly, preferably after each meal, or at least twice a day, or when new stains are apparent
Inactive ingredients
water (aqua), sorbitol, hydrated silica, dicalcium phosphate, glycerin, sodium lauryl sulfate, flavor, PVM/MA copolymer, cellulose gum, titanium
dioxide, trisodium phosphate, sodium saccharin, blue 1 (CI 42090), yellow 5 (CI 19140).
Questions or comments?
Call Toll Free 1-800-595-6230 • www.pluswhite.com
Package Labeling:
CCA Industries, Inc.
