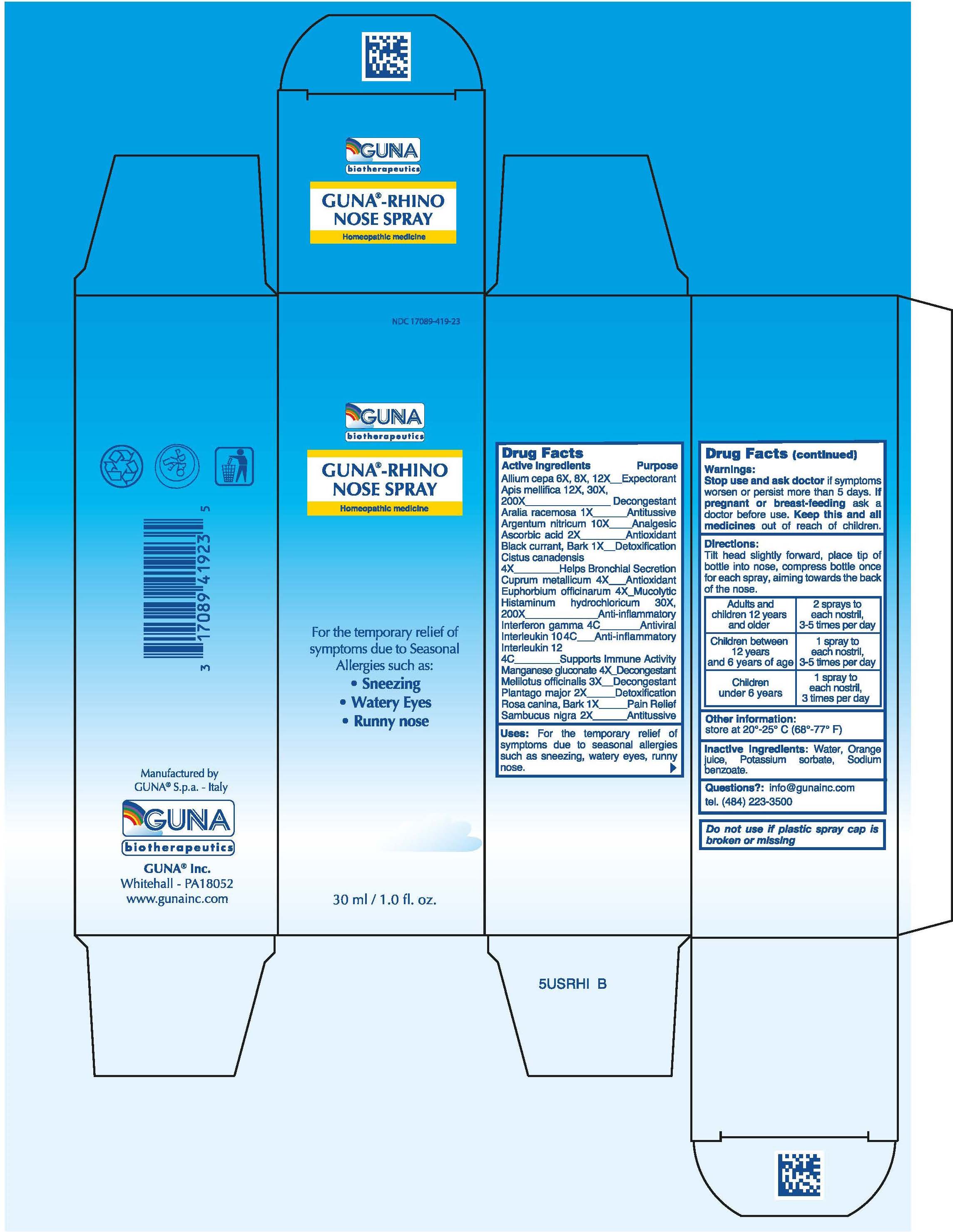
ACTIVE INGREDIENTS/PURPOSE
ALIUM CEPA 6X, 8X, 12X EXPECTORANT
APIS MELLIFICA 12x, 30X, 200X DECONGESTANT
ARALIA RACEMOSA 1X ANTITUSSIVE
ARGENTUM NITRICUM 10X ANALGESIC
ASCORBIC ACID 2X ANTIOXIDANT
BLACK CURRANT, BARK 1X DETOXIFICATION
CISTUS CANADENSIS 4X HELPS BRONCHIAL SECRETION
CUPRUM METALLICUM 4X ANTIOXIDANT
EUPHORBIUM OFFICINARUM 4X MUCOLYTIC
HISTAMINUM HYDROCHLORICUM 30X, 200X ANTI-INFLAMMATORY
INTERFERON GAMMA 4C ANTIVIRAL
INTERLEUKIN 10 4C ANTI-INFLAMMATORY
INTERLEUKIN 12 4C SUPPORTS IMMUNE ACTIVITY
MANGANESE GLUCONATE 4X DECONGESTANT
MELILOTUS OFFICINALIS 3X DECONGESTANT
PLANTAGO MAJOR 2X DETOXIFICATION
ROSA CANINA, BARK 1X PAIN RELIEF
SAMBUCUS NIGRA 2X ANTITUSSIVE
USES
For the temporary relief of symptoms due to Seasonal Allergies such as: Sneezing, Watery Eyes, Runny nose
DIRECTIONS
Adults and children 12 years and older 2 sprays to each nostril , 3-5 times per day
Children between 12 years and 6 years of age 1 spray to each nostril, 3-5 times per day
Children under 6 years 1 spray to each nostril, 3 times per day