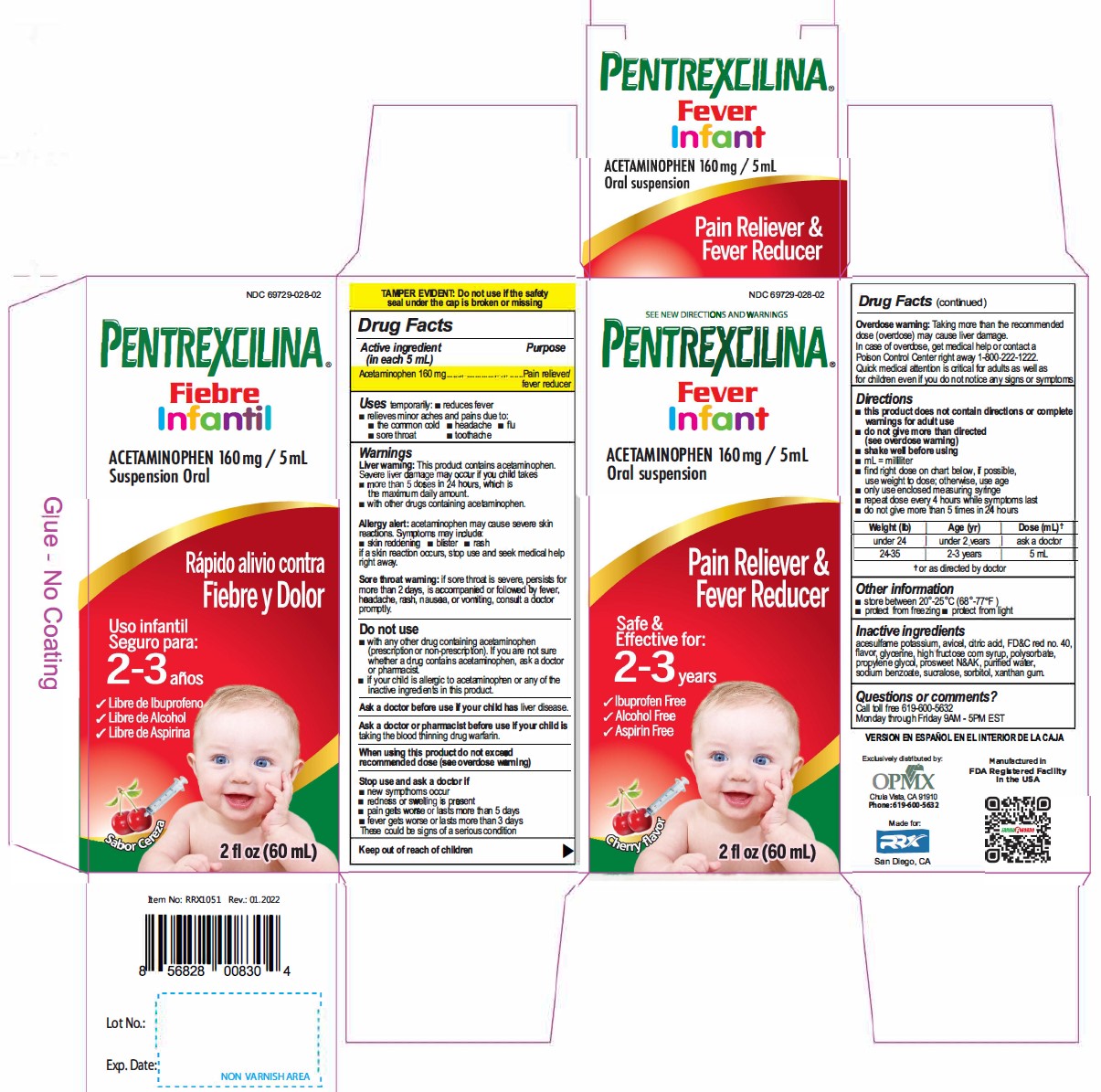
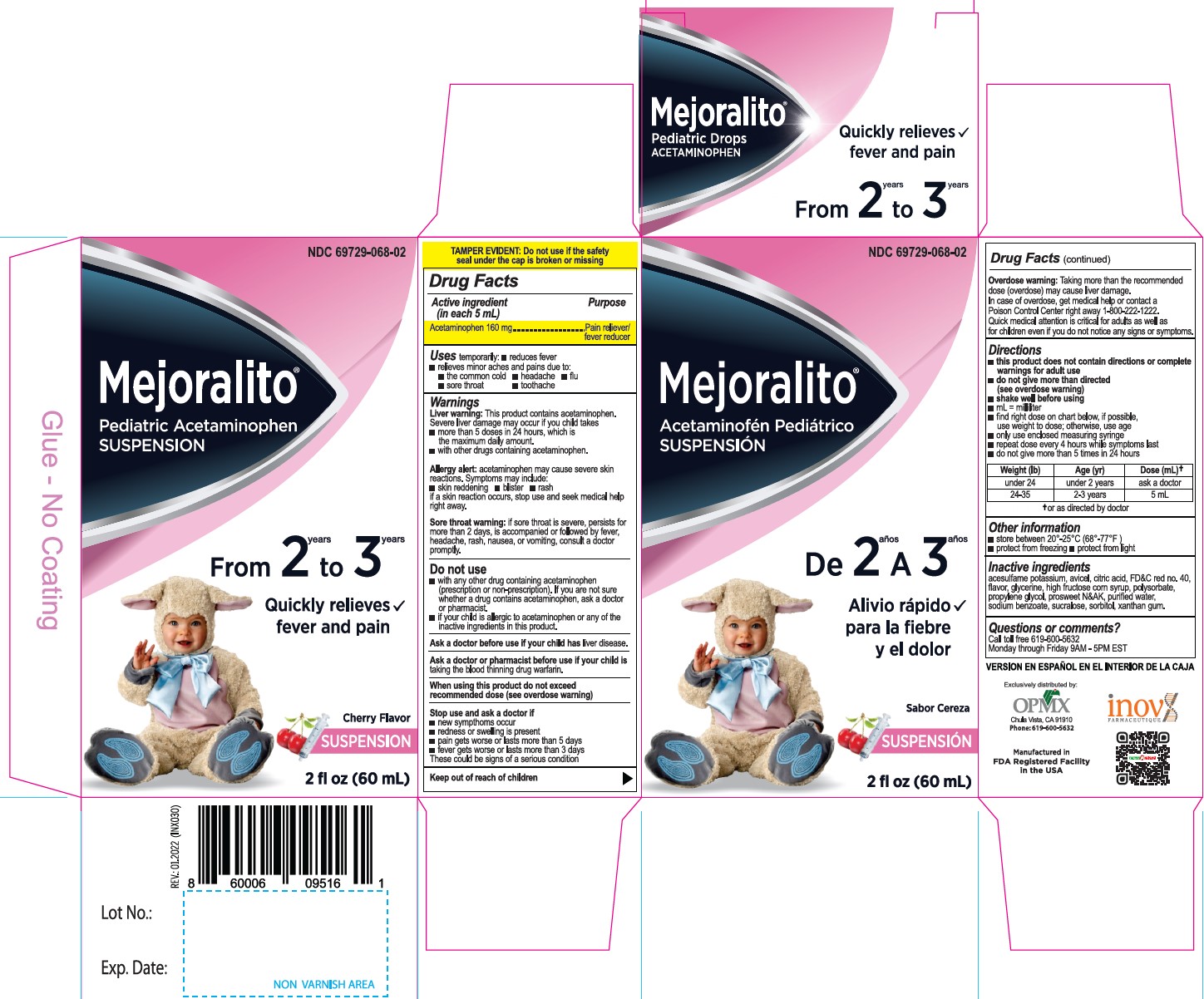
Active Ingredient(in each 5mL) Purpose
Acetaminophen 160 mg............. Pain reliever/fever reducer
Uses
temporarily:
- reduces fever
- relieves minor aches and pains due to:
- the common cold
- headache
- flu
- sore throat
- toothache
Warnings
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if your child takes takes
- more than 5 doses in 24 hours. which is the maximum daily amount.
- with other drugs containing acetaminophen.
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blister
- rash
if a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Stop use and ask a doctor if
- new sympthoms occur
- redness or swelling is present
- pain gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
These could be signs of a serious condition
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away 1-800-222-1222. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed (see overdose warning)
- shake well before using
- mL= millilitar
- find right dose on chart below, if possible, use weight to dose; otherwise, use age
- only use enclosed measuring syringe
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
|
||
|
Weight (Ib) |
Age (yr) |
Dose (mL) * |
|
under 24 |
under 2 years |
ask a doctor |
|
24-35 |
2-3 years |
5 mL |
Other information
- store between 20°-25°C (68°-77°F)
- protect from freezing
- protect from light
Questions or comments?
Call toll free 619-600-5632
Monday through Fnday 9AM - 5PM EST