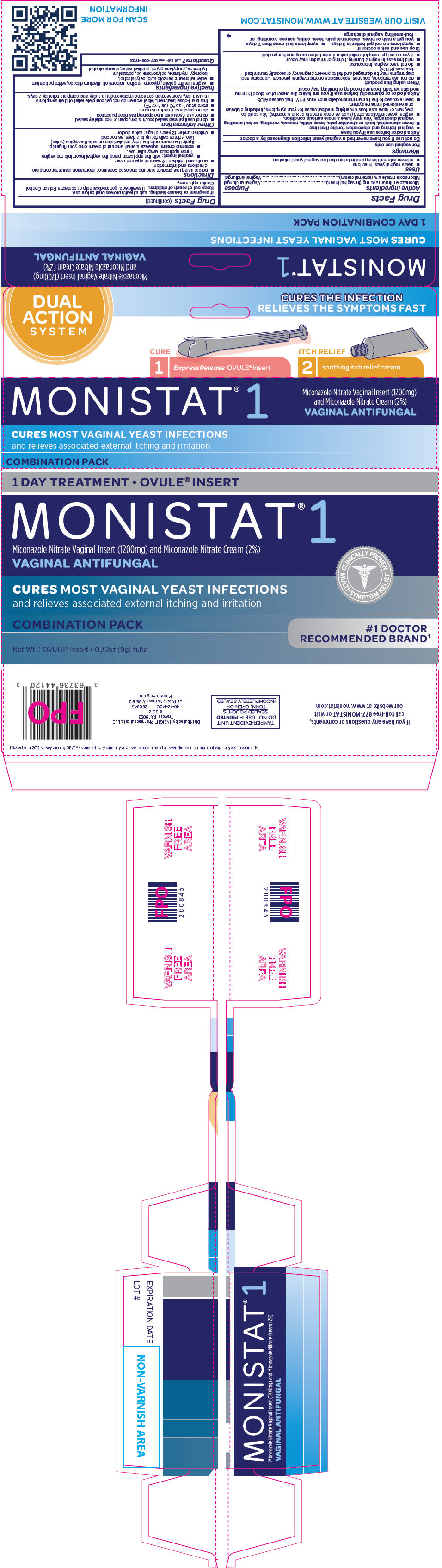
Uses
- treats vaginal yeast infections
- relieves external itching and irritation due to a vaginal yeast infection
Warnings
For vaginal use only
Ask a doctor before use if you have
- vaginal itching and discomfort for the first time
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS
Ask a doctor or pharmacist before use if you are taking the prescription blood thinning medicine warfarin, because bleeding or bruising may occur
When using this product
- do not use tampons, douches, spermicides or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted diseases (STDs).
- do not have vaginal intercourse
- mild increase in vaginal burning, itching or irritation may occur
- if you do not get complete relief ask a doctor before using another product
Directions
- before using this product read the enclosed consumer information leaflet for complete directions and information
- adults and children 12 years of age and over:
- vaginal insert: with the applicator, place the vaginal insert into the vagina. Throw applicator away after use.
- external cream: squeeze a small amount of cream onto your fingertip. Apply the cream onto the itchy, irritated skin outside the vagina (vulva). Use 2 times daily for up to 7 days, as needed.
- children under 12 years of age: ask a doctor
Other information
- do not use if printed sealed pouch is torn, open or incompletely sealed
- do not use if seal over tube opening has been punctured
- do not purchase if carton is open
- store at 20°-25°C (68°-77°F)
- this is a 1-dose treatment. Most women do not get complete relief of their symptoms in just 1 day. Most women get some improvement in 1 day and complete relief by 7 days.
Inactive ingredients
- vaginal insert: gelatin, glycerin, lecithin, mineral oil, titanium dioxide, white petrolatum
- external cream: benzoic acid, cetyl alcohol, isopropyl myristate, polysorbate 60, potassium hydroxide, propylene glycol, purified water, stearyl alcohol
PRINCIPAL DISPLAY PANEL - Kit Carton
1 DAY TREATMENT • OVULE® INSERT
MONISTAT® 1
Miconazole Nitrate Vaginal Insert (1200mg) and Miconazole Nitrate Cream (2%)
VAGINAL ANTIFUNGAL
CLINICALLY PROVEN
MULTI-SYMPTOM RELIEF
CURES MOST VAGINAL YEAST INFECTIONS
and relieves associated external itching and irritation
COMBINATION PACK
# 1 DOCTOR
RECOMMENDED BRAND†
Net Wt. 1 OVULE® Insert • 0.32oz (9g) tube