FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
ALPHANATE, (antihemophilic factor/von Willebrand factor complex [human]), is indicated for:
- Control and prevention of bleeding episodes and perioperative management in adult and pediatric patients with Factor VIII (FVIII) deficiency due to hemophilia A.
- Surgical and/or invasive procedures in adult and pediatric patients with von Willebrand Disease (VWD) in whom desmopressin (DDAVP) is either ineffective or contraindicated. It is not indicated for patients with severe VWD (Type 3) undergoing major surgery.
2 DOSAGE AND ADMINISTRATION
For intravenous injection after reconstitution only
- Treatment with ALPHANATE should be initiated under the supervision of a physician experienced in the treatment of hemophilia.
- Each vial of ALPHANATE has the antihemophilic factor (AHF) potency (FVIII:C activity) expressed in International Units (IU) FVIII/vial on the label. Additionally, ALPHANATE contains von Willebrand Factor:Ristocetin Cofactor (VWF:RCo), which is expressed in IU VWF:RCo/vial for the treatment of VWD.
2.1 Dose
Treatment and Prevention of Bleeding Episodes and Excess Bleeding During and After Surgery in Patients with Hemophilia A
- Dosage and duration of treatment depend on the severity of the FVIII deficiency, the location and extent of bleeding, presence of inhibitors, and the patient’s clinical condition. Careful control of replacement therapy is especially important in cases of major surgery or life-threatening bleeding episodes.
- Dosing requirements and frequency of dosing is calculated on the basis of an expected initial response of 2% of normal FVIII:C increase per IU FVIII:C/kg body weight administered.1The expected in vivo peak increase in FVIII level expressed as IU/dL (or % of normal) can be estimated using the following formulas:
Dosage (international units) = body weight (kg) x desired FVIII rise (IU/dL or % normal) x 0.5 (IU/kg per IU/dL)
or
IU/dL (or % of normal) = [Total Dose (IU)/body weight (kg)] x 2
- Titrate dose and frequency to the patient’s clinical response, including individualized needs, severity of the deficiency, severity of the hemorrhage, presence of inhibitors, and FVIII level desired. Patients may vary in their pharmacokinetic (e.g., half-life, in vivo recovery) and clinical responses to ALPHANATE.
- Table 1 provides dosage guidelines for the control and prevention of bleeding episodes in hemophilia A patients. Dosing should aim at maintaining a plasma factor VIII activity level at or above the plasma levels (in IU/dL or in % of normal) outlined in the table.
| Type of Bleeding | FVIII:C Level Required(% of normal) | Doses(IU/kg) | Frequency of Doses(hours) | Duration of Therapy (days) |
| Minor • Large bruises • Significant cuts or scrapes • Uncomplicated joint hemorrhage | 30 | 15 | 12 (twice daily) | Until hemorrhage stops and healing has been achieved (1–2 days). |
| Moderate • Nose, mouth and gum bleeds • Dental extractions Hematuria | 50 | 25 | 12 (twice daily) | Until healing has been achieved (2–7 days, on average). |
| Major
• Joint hemorrhage • Muscle hemorrhage • Major trauma • Hematuria • Intracranial and intraperitoneal bleeding | 80–100 | Initial: 40–50 Maintenance: 25 | 12 (twice daily) | For at least 3–5 days Until healing has been achieved for up to 10 days. Intracranial hemorrhage may require prophylaxis therapy for up to 6 months. |
| Surgery | Prior to surgery: 80–100 After surgery: 60–100 | 40–50 30–50 | Once 12 (twice daily) | Prior to surgery For the next 7–10 days, or until healing has been achieved. |
- Monitoring parameters:
- Monitor plasma FVIII levels periodically to evaluate individual patient response to the dosage regimen.
- If dosing studies have determined that a particular patient exhibits a lower/higher than expected response and shorter/longer half-life, adjust the dose and the frequency of dosing accordingly.
- Failure to achieve the expected plasma FVIII:C level or to control bleeding after an appropriately calculated dosage may be indicative of the development of an inhibitor (an antibody to FVIII:C). Quantitate the inhibitor level by appropriate laboratory procedures and document its presence. Treatment with AHF in such cases must be individualized.2
Treatment and Prevention of Excess Bleeding During and After Surgery or Other Invasive Procedures in Patients with von Willebrand Disease
- The ratio of VWF:RCo to FVIII in ALPHANATE varies by lot, so with each new lot, check IU VWF:RCo/vial to ensure accurate dosing.
- Dosage and duration of treatment depend on the severity of the VWF deficiency, the location and extent of bleeding, and the patient’s clinical condition. Careful control of replacement therapy is especially important in cases of major surgery or life-threatening bleeding episodes.
- The median incremental in vivo recoveries of VWF:RCo and FVIII:C were 3.12 (IU/dL)/(IU/kg) [mean, 3.29 ± 1.46 (IU/dL)/(IU/kg); range: 1.28 to 5.73 (IU/dL)/(IU/kg)] for VWF:RCo and 1.95 (IU/dL)/(IU/kg) [mean, 2.13 ± 0.58 (IU/dL)/(IU/kg); range: 1.33 to 3.32 (IU/dL)/(IU/kg)] for FVIII:C.
- Table 2 provides dosing guidelines for pediatric and adult patients with von Willebrand Disease.3-6
|
a The therapeutic goal is referenced in the NHLBI Guidelines.7 |
||
|
b The safety parameter is extracted from Mannucci 2009.8 |
||
| Minor Surgery/Bleeding | ||
| Parameter | VWF:RCo | Target
FVIII:C Activity Levels |
| Pre-operative/pre-procedure dose: | Adults: 60 IU VWF:RCo/kg body weight. Pediatrics: 75 IU VWF:RCo/kg body weight. |
40-50 IU/dL |
| Maintenance dose: | Adults: 40 to 60 IU VWF:RCo/kg body weight at 8 to 12 hour intervals as clinically needed for 1-3 days. Pediatrics: 50 to 75 IU VWF:RCo/kg body weight at 8 to 12 hour intervals as clinically needed for 1-3 days. |
40-50 IU/dL |
| Therapeutic Goal (Trough)a : | >50 IU/dL | >50 IU/dL |
| Safety Monitoring: | Peak and trough at least once daily | Peak and trough at least once daily |
| Safety Parameterb : | Should not exceed 150 IU/dL | Should not exceed 150 IU/dL |
| Major Surgery/Bleeding | ||
| Parameter | VWF:RCo | Target
FVIII:C Activity Levels |
| Pre-operative/pre-procedure dose: | Adults: 60 IU VWF:RCo/kg body weight. Pediatrics: 75 IU VWF:RCo/kg body weight. | 100 IU/dL |
| Maintenance dose: | Adults: 40 to 60 IU VWF:RCo/kg body weight at 8 to 12 hour intervals as clinically needed for at least 3-7 days. Pediatrics: 50 to 75 IU VWF:RCo/kg body weight at 8 to 12 hour intervals as clinically needed for at least 3-7 days. | 100 IU/dL |
| Therapeutic Goal (Trough)a : | >50 IU/dL | >50 IU/dL |
| Safety Monitoring: | Peak and trough at least daily | Peak and trough at least daily |
| Safety Parameterb: | Should not exceed 150 IU/dL | Should not exceed 150 IU/dL |
2.2 Reconstitution
- Always use aseptic technique.
- Ensure that concentrate (ALPHANATE) and diluent (Sterile Water for Injection, USP) are at room temperature (but not above 37 ˚C) before reconstitution.
- Remove the plastic flip off cap from the diluent vial.
- Gently swab the exposed stopper surface with a cleansing agent such as alcohol trying to avoid leaving any excess cleansing agent on the stopper.
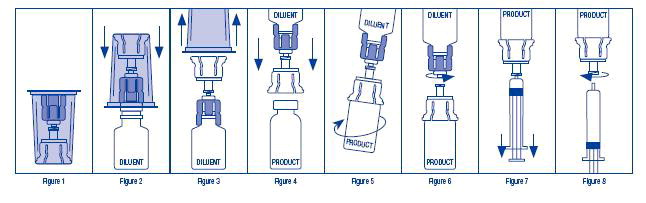
- Open the Mix2Vial package by peeling away the lid (Figure 1). Leave the Mix2Vial in the clear outer packaging.
- Place the diluent vial upright on an even surface and hold the vial tight and pick up the Mix2Vial in its clear outer packaging. Holding the diluent vial securely, push the blue end of the Mix2Vial vertically down through the diluent vial stopper (Figure 2).
- While holding onto the diluent vial, carefully remove the clear outer packaging from the Mix2Vial set, ensuring the Mix2Vial remains attached to the diluent vial (Figure 3).
- Place the product vial upright on an even surface, invert the diluent vial with the Mix2Vial attached.
- While holding the product vial securely on a flat surface, push the clear end of the Mix2Vial set vertically down through the product vial stopper (Figure 4). The diluent will automatically transfer out of its vial into the product vial.
NOTE: If the Mix2Vial is connected at an angle, the vacuum may be released from the product vial and the diluent will not transfer into the product vial.
- With the diluent and product vials still attached to the Mix2Vial, gently swirl the product vial to ensure the product is fully dissolved (Figure 5). Reconstitution requires less than 5 minutes. Do not shake the vial.
- Disconnect the Mix2Vial into two separate pieces (Figure 6) by holding each vial adapter and twisting counterclockwise. After separating, discard the diluent vial with the blue end of the Mix2Vial.
- Draw air into an empty, sterile syringe. Keeping the product vial upright with the clear end of the Mix2Vial attached, screw the disposable syringe onto the luer lock portion of the Mix2Vial device by pressing and twisting clockwise. Inject air into the product vial.
- While keeping the syringe plunger depressed, invert the system upside down and draw the reconstituted product into the syringe by pulling the plunger back slowly (Figure 7).
- When the reconstituted product has been transferred into the syringe, firmly hold the barrel of the syringe and the clear vial adapter (keeping the syringe plunger facing down) and unscrew the syringe from the Mix2Vial (Figure 8). Hold the syringe upright and push the plunger until no air is left in the syringe. Attach the syringe to a venipuncture set.
NOTE: If the same patient is to receive more than one vial of concentrate, the contents of two vials may be drawn into the same syringe through a separate unused Mix2Vial set before attaching to the venipuncture set.
- When reconstitution procedure is strictly followed, a few small particles may occasionally remain. The Mix2Vial set will remove particles and the labeled potency will not be reduced.
- Discard all reconstitution equipment after use into the appropriate safety container. Do not reuse.
- Use the prepared drug as soon as possible within 3 hours after reconstitution.
2.3 Administration
For intravenous use after reconstitution only
- Inspect parenteral drug products visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Do not refrigerate after reconstitution. Store reconstituted ALPHANATE at room temperature (not to exceed 30 °C) prior to administration, but administer intravenously within three hours.
- Use plastic disposable syringes.
- Do not administer ALPHANATE at a rate exceeding 10 mL/minute.
- Discard any unused contents into the appropriate safety container.
3 DOSAGE FORMS AND STRENGTHS
ALPHANATE is available as a lyophilized powder for intravenous injection after reconstitution. It is available in the following potencies:
250 IU FVIII/5 mL single dose vial
500 IU FVIII/5 mL single dose vial
1000 IU FVIII/10 mL single dose vial
1500 IU FVIII/10 mL single dose vial
2000 IU FVIII/10 mL single dose vial
4 CONTRAINDICATIONS
ALPHANATE is contraindicated in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product or its components [see Adverse Reactions (6)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Anaphylaxis and severe hypersensitivity reactions are possible with ALPHANATE. Early signs of allergic reactions, which can progress to anaphylaxis, may include angioedema, chest tightness, hypotension, rash, nausea, vomiting, paresthesia, restlessness, wheezing and dyspnea. Discontinue use of ALPHANATE if hypersensitivity symptoms occur, and initiate appropriate treatment.
5.2 Neutralizing Antibodies
Development of procoagulant activity-neutralizing antibodies (inhibitors) has been detected in patients receiving FVIII-containing products. Carefully monitor patients treated with AHF products for the development of FVIII inhibitors by appropriate clinical observations and laboratory tests. No specific studies have been conducted with ALPHANATE to evaluate inhibitor formation. If expected plasma FVIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an appropriate assay that measures FVIII inhibitor concentration.
5.3 Thromboembolic Events
Thromboembolic events have been reported in von Willebrand Disease patients receiving replacement therapy with Antihemophilic Factor/von Willebrand Factor Complexes, especially in those with known risk factors for thrombosis including but not limited to elderly age, previous thrombosis, metabolic syndrome, cancer, surgery, oral contraceptive and hormone therapy, diabetes, hypertension, hyperlipidemia, smoking, and pregnancy.9 Monitor plasma levels of VWF:RCo and FVIII activities to avoid sustained excessive VWF and FVIII activity levels (greater than 150 IU/dL), which may increase the risk of thrombotic events, during continued treatment of replacement therapy with Antihemophilic Factor/von Willebrand Factor Complexes. Consider antithrombotic measures in VWD patients at risk for thrombosis [see Adverse Reactions (6)].
5.4 Intravascular Hemolysis
ALPHANATE contains blood group specific isoagglutinins. Monitor the patient for signs of intravascular hemolysis and decreasing hematocrit when large and/or frequent doses of Antihemophilic Factor/von Willebrand Factor Complexes are required in patients of blood groups A, B, or AB, as cases of acute hemolytic anemia, increased bleeding tendency or hyperfibrinogenemia have been reported. These events typically subside after cessation of the factor concentrate infusion.10 Consider alternative therapy should this condition worsen despite discontinuation of ALPHANATE.
5.5 Vasomotor Reactions
Rapid administration of a FVIII concentrate may result in vasomotor reactions. Do not administer ALPHANATE at a rate exceeding 10 mL/minute.
5.6 Transmissible Infectious Agents
Because ALPHANATE is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob Disease (vCJD) agent and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain virus infections, and by inactivating and/or removing certain viruses during manufacturing. [see Description (11)].
5.7 Monitoring Laboratory Tests
Monitor for development of FVIII and VWF inhibitors. Perform appropriate assays to determine if FVIII and/or VWF inhibitor(s) are present if bleeding is not controlled with expected dose of ALPHANATE.
Monitor plasma levels of VWF:RCo and FVIII activities to avoid sustained excessive VWF and FVIII activity levels (greater than 150 IU/dL), which may increase the risk of thrombotic events, particularly in patients with known risk factors.
6 ADVERSE REACTIONS
Serious adverse drug reactions (ADRs) observed in patients receiving ALPHANATE include anaphylaxis/hypersensitivity reactions. Thromboembolic events also have been observed in patients receiving ALPHANATE for VWD [see Warnings and Precautions (5.3)].
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse drug reaction (ADR) rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
Hemophilia A
In a prospective clinical study with ALPHANATE, 23 subjects were exposed to 1217 infusions (median=42, range 2-160). The total number of exposure days was 1133, and the total number of months on study across all subjects was 234 (19.5 subject years). No ADRs or inhibitors to FVIII were reported during the study.
von Willebrand Disease
In the prospective clinical study of ALPHANATE[using both ALPHANATE Solvent Detergent (A-SD, a previous generation product) and ALPHANATE Solvent Detergent/Heat Treated (A-SD/HT, the current generation product)] in subjects with von Willebrand Disease, ADRs occurred in 5 of 36 subjects (13.9%) treated with ALPHANATE.
Sixty-one total ADRs were reported in 204 infusions. The majority of ADRs were rated as mild (55 of 61 [90.2%]). Six ADRs (9.8%) were rated as moderate. No reactions rated as serious were reported. The adverse drug reaction grading scale is defined as follows:
- Mild: the event was noted but the administration of the compound was not interrupted; the event resolved spontaneously or no treatment was required beyond administration of nonprescription analgesics.
- Moderate: the administration of the compound was not necessarily interrupted; the event required momentary treatment with prescription drugs and produced no sequelae.
Overall, the proportion of infusions associated with ADRs was 14 of 204 infusions (6.9%).
The most common ADRs reported (> 1% of infusions) were pruritus, headache, backpain, paresthesia, respiratory distress, facial edema, pain, rash, and chills.
One incident of pulmonary embolism was reported that was considered to have a possible relationship to the product. This subject received a dose of 60 IU VWF:RCo/kg body weight and the FVIII:C level achieved was 290%.
In the retrospective study conducted to determine the efficacy and safety of ALPHANATE (A-SD/HT) in a surgical or invasive procedure setting as perioperative prophylaxis against excessive bleeding, [see Clinical Studies (14)], 3 out of 39 subjects (7.7%) experienced 6 adverse drug reactions. Four were considered mild and 2 were considered moderate. No subject discontinued their treatment due to an adverse drug reaction. The adverse drug reactions were pruritus, paresthesia (2 events) and hemorrhage (all considered mild), and one event each of moderate hematocrit decrease and orthostatic hypotension.
One adverse drug reaction (pain) related to the treatment with heat-treated ALPHANATE (A-SD/HT) was reported in the four pediatric subjects with von Willebrand Disease during the course of the prospective study and in none of the five pediatric subjects in the retrospective clinical study.
6.2 Post-Marketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The most common post-marketing ADRs reported include allergic/hypersensitivity reactions, nausea, fever, joint pain, fatigue, and infusion site pain.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with ALPHANATE. It is also not known whether ALPHANATE can cause fetal harm when administered to a pregnant woman or affect reproductive capacity. ALPHANATE should be given to a pregnant woman only if clearly needed.
8.4 Pediatric Use
Hemophilia A
A total of 21 children (ages 7-16) were included in clinical trials with ALPHANATE. Subjects received ALPHANATE weekly for prophylaxis or suspected bleeds. They were successfully treated for 1499 bleeding episodes or as prophylaxis to prevent them (e.g. pain in the joint). The median number of units needed to treat the bleeds was 420 IU, with a range of 210 to 1620 IU. Adult and pediatric subjects did not differ in their response to treatment.
Von Willebrand Disease
The hemostatic efficacy of ALPHANATE has been studied in 20 pediatric subjects (ages 7-18) with VWD. Based on the data from a subset of these subjects, age had no effect on the pharmacokinetics of VWF:RCo. Adult and pediatric subjects did not differ in their response to treatment.
11 DESCRIPTION
ALPHANATE, (antihemophilic factor/von Willebrand factor complex [human]), is a sterile, lyophilized concentrate of FVIII (AHF) and von Willebrand Factor (VWF).
ALPHANATE is prepared from pooled human plasma by cryoprecipitation of FVIII, fractional solubilization, and further purification employing heparin-coupled, cross-linked agarose which has an affinity to the heparin binding domain of VWF/FVIII:C complex. The product is treated with a mixture of tri-n-butyl phosphate (TNBP) and polysorbate 80 to inactivate enveloped viruses. The product is also subjected to an 80 °C heat treatment step for 72 hours to inactivate enveloped and non-enveloped viruses. However, no procedure has been shown to be totally effective in removing viral infectivity from coagulation factor products.
ALPHANATE is labeled with the antihemophilic factor potency (FVIII:C activity) in International Units (IU) FVIII/vial and with VWF:RCo activity expressed in IU VWF:RCo/vial. The activities are referenced to their respective international standards established by the World Health Organization. One IU of FVIII or one IU of VWF:RCo is approximately equal to the amount of FVIII or VWF:RCo activity in 1 mL of freshly-pooled human plasma.
ALPHANATE contains human albumin as a stabilizer, resulting in a final container concentrate with a specific activity of at least 5 FVIII:C IU/mg total protein. ALPHANATE contains no preservatives.
The composition of ALPHANATE after reconstitution is as follows:
|
a Supplied in a separate diluent vial |
||||||
| Name of Ingredients | Nominal Composition | Units/Container | ||||
| Factor VIII | 250 | 500 | 1000 | 1500 | 2000 | IU |
| von Willebrand Factor | > 400 | > 400 | > 400 | > 400 | > 400 | IU per 1000 IU Factor VIII |
| Albumin (Human) | 25 | 25 | 50 | 50 | 50 | mg |
| Arginine | 90 | 90 | 175 | 175 | 175 | mg |
| Histidine | 20 | 20 | 40 | 40 | 40 | mg |
| Water for Injectiona | 5 | 5 | 10 | 10 | 10 | mL |
Viral Reduction Capacity
The results of virus validation studies performed to determine virus reduction factors associated with several steps in the manufacturing process of ALPHANATE are summarized in Table 3.
In vitro inactivation studies to evaluate the solvent detergent treatment (0.3% Tri-n-butyl Phosphate and 1.0% Polysorbate 80) step in the manufacture of ALPHANATE were conducted to assess the capability of the step to inactivate enveloped viruses, such as Human Immunodeficiency viruses (HIV), as well as marker viruses such as Sindbis virus (SIN, a model for Hepatitis C virus), Vesicular Stomatitis virus (VSV, a model for large, enveloped RNA virus), Bovine Herpes virus (BHV, a model for Hepatitis B virus) and Bovine Viral Diarrhea virus (BVD, a model for Hepatitis C virus). In vitro inactivation studies to evaluate the dry heat treatment (80 °C, 72 hours) step in the manufacture of ALPHANATE were conducted to assess the capability of the step to inactivate both enveloped and non-enveloped viruses, such as Hepatitis A virus (HAV), human Poliovirus Sabin type 2 (POL, a model for HAV), Canine Parvovirus (CPV, a model for Parvovirus B19), BHV and BVD. Other steps in the manufacturing process of ALPHANATE (precipitation with 3.5% polyethylene glycol (PEG), heparin affinity chromatography and lyophilization) were also evaluated for virus elimination capability using several enveloped and non-enveloped viruses as shown in Table 3.
|
Virus (Model Virus for) | 3.5% PEG
Precipitation | Solvent- Detergent | Column
Chromatography | Lyophilization | Dry Heat Cycle
(80 °C, 72 hr) | Total Log
Reduction |
| BHV (HBV) | < 1.0 | ≥ 8.0 | 7.6 | 1.3 | 2.1 | ≥ 19.0 |
| BVD (HCV) | < 1.0 | ≥ 4.5 | < 1.0 | < 1.0 | ≥ 4.9 | ≥ 9.4 |
| POL (HAV) | 3.3 | – | < 1.0 | 3.4 | ≥ 2.5 | ≥ 9.2 |
| CPV (B19) | 1.2 | – | < 1.0 | < 1.0 | 4.1 | 5.3 |
| VSV | – | ≥ 4.1 | – | – | – | ≥ 4.1 |
| SIN (HCV) | – | ≥ 4.7 | – | – | – | ≥ 4.7 |
| HIV–1 | < 1.0 | ≥ 11.1 | ≥ 2.0 | – | – | ≥ 13.1 |
| HIV–2 | – | ≥ 6.1 | – | – | – | ≥ 6.1 |
| HAV | – | – | – | 2.1 | ≥ 5.8 | ≥ 7.9 |
Additionally, the manufacturing process was investigated for its capacity to decrease infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the vCJD and CJD agents.
Several of the individual production steps in ALPHANATE manufacturing process have been shown to decrease TSE infectivity of an experimental model agent.11 TSE reduction steps include: 3.5% polyethylene glycol precipitation (3.23 log10), affinity chromatography (3.50 log10) and saline precipitation (1.36 log10). These studies provide reasonable assurance that low levels of CJD/vCJD agent infectivity, if present in the starting material, would be removed.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ALPHANATE contains antihemophilic factor (FVIII) and von Willebrand factor (VWF), constituents of normal plasma. FVIII is an essential cofactor in activation of factor X leading to formation of thrombin and fibrin. VWF promotes platelet aggregation and platelet adhesion on damaged vascular endothelium; it also serves as a stabilizing carrier protein for the procoagulant protein FVIII.12, 13
After administration, ALPHANATE temporarily replaces the missing coagulation factor VIII and von Willebrand factor needed for effective hemostasis.
12.3 Pharmacokinetics
Pharmacokinetics in Hemophilia A
Following the administration of ALPHANATE during clinical trials, the mean in vivo half-life of FVIII observed in 12 adult subjects with severe hemophilia A was 17.9 ± 9.6 hours. In this same study, the in vivo recovery was 96.7 ± 14.5% at 10 minutes post-infusion. Recovery at 10 minutes post-infusion was also determined as 2.4 ± 0.4 IU FVIII rise/dL plasma per IU FVIII infused/kg body weight.
Pharmacokinetics in von Willebrand Disease (VWD)
A pharmacokinetic crossover study was conducted in 14 non-bleeding subjects with VWD (1 type 1, 2 type 2A, and 11 type 3) comparing the pharmacokinetics of ALPHANATE (A-SD/HT) and an earlier formulation, ALPHANATE (A-SD). Subjects received, in random order at least seven days apart, a single intravenous dose of each product, 60 IU VWF:RCo/kg (75 IU VWF:RCo/kg in subjects younger than 18 years of age). Pharmacokinetic parameters were similar for the two products and indicated that they were biochemically equivalent. Pharmacokinetic analysis of ALPHANATE (A-SD/HT) in the 14 subjects revealed the following results: the median plasma levels (% normal) of VWF:RCo rose from 10 IU/dL (range: 10 to 27 IU/dL) at baseline to 206 IU/dL (range: 87 to 440 IU/dL) 15 minutes post-infusion; median plasma levels of FVIII:C rose from 5 IU/dL (range: 2 to 114 IU/dL) to 206 IU/dL (range: 110 to 421 IU/dL). The median bleeding time (BT) prior to infusion was 30 minutes (mean, 28.8 ± 4.41 minutes; range: 13.5 to 30 minutes), which shortened to 10.38 minutes (mean, 10.4 ± 3.2 minutes; range: 6 to 16 minutes) 1 hour post-infusion.
Following infusion of ALPHANATE (A-SD/HT), the median half-lives for VWF:RCo, FVIII:C and VWF:Ag were 6.91 hours (range: 3.8 to 16.22 hours), 20.92 hours (range: 7.19 to 32.2 hours), and 12.8 hours (range: 10.34 to 17.45 hours), respectively. The median incremental in vivo recoveries of VWF:RCo and FVIII:C were 3.12 (IU/dL)/(IU/kg) [range: 1.28 to 5.73 (IU/dL)/(IU/kg)] for VWF:RCo and 1.95 (IU/dL)/(IU/kg) [range: 1.33 to 3.32 (IU/dL)/(IU/kg)] for FVIII:C.
The pharmacokinetic data in VWD are summarized in Table 4.
| Parameter
| Plasma VWF:RCo
(Mean ± SD) | Plasma FVIII:C
(Mean ± SD) | Plasma VWF:Ag
(Mean ± SD) |
| Number of patients | 14 | 14 | 14 |
| Mean plasma levels (IU/dL) | |||
| Baseline | 11.86 ± 4.97 | 21.00 ± 33.83 | – |
| 15 minutes post-infusion | 215.50 ± 101.70 | 215.29 ± 94.26 | – |
| T½ (Half-life in hours) | 7.67 ± 3.32 | 21.58 ± 7.79 | 13.06 ± 2.20 |
| Incremental in vivo recovery in (IU/dL)/(IU/kg) | 3.29 ± 1.46 | 2.13 ± 0.58 | – |
Following infusion of both ALPHANATE (A-SD) and ALPHANATE (A-SD/HT), an increase in the size of VWF multimers was seen and persisted for at least 24 hours. The shortening of the BT was transient, lasting less than 6 hours following treatment and did not correlate with the presence of large and intermediate size VWF multimers.14
14 CLINICAL STUDIES
In a prospective, multi-center clinical study, 37 subjects with VWD (6 Type 1, 19 Type 2, 12 Type 3) underwent 59 surgical procedures for which ALPHANATE (A-SD) or ALPHANATE (A-SD/HT) was administered [21 subjects received ALPHANATE (A-SD), 18 received ALPHANATE (A-SD/HT), and 2 received both products] for bleeding prophylaxis (see Table 5). An initial pre-operative infusion of 60 IU VWF:RCo/kg (75 IU VWF:RCo/kg for subjects less than 18 years of age), was administered one hour before surgery. A blood sample was obtained 15 minutes after the initial infusion for the determination of the plasma FVIII:C level. The level had to equal or exceed 100% of normal for an operation to proceed. No cryoprecipitate or alternative FVIII product was administered during these surgical procedures. Platelets were required in two subjects. The protocol permitted intra-operative infusions of ALPHANATE (A-SD) and ALPHANATE (A-SD/HT) at 60 IU VWF:RCo/kg (75 IU VWF:RCo/kg for subjects less than 18 years of age) to be administered as required according to the judgment of the investigator.
|
^ Two subjects received both preparations; the total number of subjects is therefore less than the sum of the columns. |
|||
| Parameter | Treatment with Alphanate | Total | |
| Type of Surgical Procedure | A-SD | A-SD/HT | |
| Number of Subjects | 21 | 18 | 37^ |
| Dental | 14 | 6 | 20 |
| Dermatologic | 1 | 1 | 2 |
| Gastrointestinal | 4 | 4 | 8 |
| Gastrointestinal (diagnostic) | 6 | 0 | 6 |
| Genitourinary | 0 | 2 | 2 |
| Gynecologic | 2 | 1 | 3 |
| Head and neck | 1 | 1 | 2 |
| Orthopedic | 4 | 3 | 7 |
| Vascular | 3 | 6 | 9 |
| Total number of procedures | 35 | 24 | 59 |
Post-operative infusions at doses of 40 to 60 IU VWF:RCo/kg (50 to 75 IU VWF:RCo/kg for pediatric subjects) were administered at 8 to 12-hour intervals until healing had occurred. For maintenance of secondary hemostasis (after primary hemostasis was achieved), the dose was reduced after the third post-operative day [see Dosage and Administration (2.2)].
Overall, in the surgical procedures using either product, the BT at 30 minutes post-infusion was fully corrected in 18 (32.7%) cases, partially corrected in 24 (43.6%) cases, not corrected in 12 (21.8%) cases, and was not done in one case (1.8%). Overall, the mean blood loss was lower than predicted prospectively.
Surgical infusion summary data are included in Table 6.
|
* Two subjects received both products |
|||
| Parameter | A-SD | A-SD/HT | Total |
| Number of patients | 21 | 18 | 37* |
| Number of surgical procedures | 35 | 24 | 59 |
| Median number of infusions per surgical procedure (range) | 3 (1-13) | 4 (1 – 18) | 4 (1-18) |
| Median dosage IU VWF:RCo/kg | |||
| Infusion #1 (range) | 59.8 (19.8-75.1) | 59.9 (40.6 – 75.0) | 59.9 (19.8-75.1) |
| Infusion ≥ #2 combined (range) | 40.0 (4.5-75.1) | 40.0 (10.0 – 63.1) | 40.0 (4.5-75.1) |
Additionally, surgical procedures using ALPHANATE SD/HT only were categorized as major, minor or invasive procedures according to definitions used in the study. The outcome of each surgery was evaluated according to a clinical rating scale (excellent, good, poor or none) and was considered successful if the outcome was excellent or good.
Study results also were evaluated independently by two referees with clinical experience in this field in the same way (surgery categorization and outcome of each surgery according to a clinical rating scale). There was a high level of agreement between the referee evaluations and the analyzed outcome data, with a decrease of only a single success in achieving hemostasis (21/24 [referees evaluation] vs. 22/24 [investigators evaluation]).
A retrospective, multi-center study was performed to assess the efficacy of ALPHANATE (A-SD/HT) as replacement therapy in preventing excessive bleeding in subjects with congenital VWD undergoing surgical or invasive procedures, for whom DDAVP was ineffective or inadequate. A total of 61 surgeries/procedures in 39 subjects were evaluated.15
Of the 39 subjects, 18 had Type 1 VWD (46.2%); 12 subjects (30.8%) had Type 2 VWD, and 9 subjects (23.1%) had Type 3 VWD. Median age was 40 years; approximately one-half of the subjects were male.
The primary efficacy variable was the overall treatment outcome for each surgical or invasive procedure, as rated by the investigator using a 4-point verbal rating scale (VRS): “excellent,” “good,” “poor,” or “none (no indication of efficacy).” The categorization of the replacement treatment outcome was based upon the investigator’s clinical experience and defined in Table 7.
| * The efficacy assessment period included the entire perioperative period. | ||||
| Rating | Clinical Efficacy* | |||
| Hemostasis | Dosing | |||
| Excellent | Hemostasis not different from that expected for subjects without known bleeding disorders. | No upward dosage adjustment for ALPHANATE replacement therapy. | ||
| Good | Hemostasis slightly inferior from that expected for subjects without known bleeding disorders but judged as not clinically relevant. | Minor upward dosage adjustment for ALPHANATE replacement therapy. | ||
| Poor | Less hemostasis than expected for subjects without known bleeding disorders attributed to vWD despite ALPHANATE replacement therapy. | Relevant upward dosage adjustment for ALPHANATE replacement therapy. No need for alternative therapy. |
||
| None | Severe bleeding attributed to vWD despite ALPHANATE replacement therapy. | Relevant upward dosage adjustment for ALPHANATE replacement therapy and/or need for alternative unexpected therapy. | ||
In addition, an independent referee committee was convened to evaluate the efficacy outcomes. More than 90% of the surgical outcomes received an investigator and referee’s overall and daily rating of “effective” (“excellent” or “good”) in achieving hemostasis/preventing bleeding.
The majority of ratings were considered “excellent” (≥ 81.3% in each VWD type). Nine Type 3 subjects underwent 1 major and 15 minor procedures. Two procedures (1 major and 1 minor) in 1 subject with Type 3 VWD received an overall efficacy rating of “none,” and one minor procedure in a subject with Type 2 VWD received an overall efficacy rating of “poor.”
15 REFERENCES
- Srivastava, A., Brewer, A.K., et al. WFH Guidelines: Guidelines for the management of hemophilia. Haemophilia 2013; 19, e1-e47.
- Kempton, C.L., White, G.C. How we treat a hemophilia A patient with a factor VIII inhibitor. Blood 2009; 113:11-17.
- Federici, A.B., Baudo, F., Caracciolo, C., Mancuso, G., Mazzucconi, M.G., Musso, R., Schinco, P.C., Targhetta. R., Mannucci, P.M. Clinical efficacy of highly purified, doubly virus-inactivated factor VIII/von Willebrand factor concentrate (Fanhdi) in the treatment of von Willebrand disease: a retrospective clinical study. Haemophilia 2002; 8:761-767.
- Federici, A.B. Management of von Willebrand disease with factor VIII/von Willebrand factor concentrates: results from current studies and surveys. Blood Coagul Fibrinolysis 2005; 16(Suppl 1):S17-S21.
- Mannucci, P.M. How I treat patients with von Willebrand disease. Blood 2001; 97:1915-1919.
- Mannucci, P.M. Treatment of von Willebrand’s Disease. N Engl J Med 2004; 351:47-58.
- Nichols, W.L. et al.; NHLBI VWD Expert Panel. The Diagnosis, Evaluation, and Management of von Willebrand Disease. US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute 2007; NIH No. 08-5832.
- Mannucci, P.M., Franchini, M., Castaman, G., Federici, A.B.; Italian Association of Haemophilia Centres. Evidence-based recommendations on the treatment of von Willebrand disease in Italy. Blood Transfus 2009; 7:117-126.
- Coppola, A., Franchini, M., Makris M. Santagostino, E. Minno, G.DI, Mannucci, P.M. Thrombotic adverse events to coagulation factor concentrates for treatment of patients with haemophilia and von Willebrand disease: a systematic review of prospective studies. Haemophilia 2012; 18, e173-e187.
- Soni, N.S., Patel A.R., Vohra, R.M., Shah P.C. Hemophiliac with Hemolytic Anemia resulting from Factor VIII Concentrate. Acta Haemato 1977; 58:294-297.
- Diez, J.M., Caballero, S., Belda, P., Otegui, M., Gajardo, R., Jorquera, J.I. Elimination capacity of a TSE-model agent in the manufacturing process of Alphanate/Fanhdi, a human factor VIII/VWF complex concentrate. Haemophilia 2009; 15(6):1249-1257.
- Hoyer, L.W. The Factor VIII Complex: Structure and Function. Blood 1981; 58:1-13.
- Meyer, D., Girma, J-P. von Willebrand Factor: Structure and Function. Thrombosis and Haemostasis 1993; 70:99-104.
- Mannucci, P.M., Chediak, J., Hanna, W. Byrnes, J.J., Kessler, C.M, Ledford, M., Retzios, A.D., Kapelan, B.A., Gallagher, P., Schwartz, R.S., and the Alphanate Study Group. Treatment of von Willebrand’s Disease (VWD) with a high purity factor VIII concentrate: Dissociation between correction of the bleeding time (BT), VWF multimer pattern, and treatment efficacy. Blood 1999; 94 (Suppl 1, Part 2 of 2):98b.
- Rivard, G.E., Aledort, L., et al. Efficacy of factor VIII/von Willebrand factor concentrate Alphanate in preventing excessive bleeding during surgery in subjects with von Willebrand disease. Haemophilia 2008; 14, 271-275.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ALPHANATE is supplied in sterile, lyophilized form in a single dose vial with a vial of diluent (Sterile Water for Injection, USP) and a Mix2Vial filter transfer set. IU activity of FVIII and VWF:RCo are stated on the carton and label of each vial.
ALPHANATE is available in the following potencies and color coded based upon assay on the carton and label as follows:
| Potency | NDC | Assay Color Code |
| 250 IU FVIII/5 mL single dose vial | 68516-4611-1 or 68516-4616-1 | 250 IU FVIII Range – gray box |
| 500 IU FVIII/5 mL single dose vial | 68516-4612-1 or 68516-4617-1 | 500 IU FVIII Range - green box |
| 1000 IU FVIII/10 mL single dose vial | 68516-4613-2 or 68516-4618-2 | 1000 IU FVIII Range – blue box |
| 1500 IU FVIII/10 mL single dose vial | 68516-4614-2 or 68516-4619-2 | 1500 IU FVIII Range – orange box |
| 2000 IU FVIII/10 mL single dose vial | 68516-4615-2 or 68516-4620-2 | 2000 IU FVIII Range - magenta box |
17 PATIENT COUNSELING INFORMATION
Advise the patient:
- To contact their healthcare provider or go to the emergency department right away if a hypersensitivity reaction occurs. Early signs of hypersensitivity reactions may include rash, hives, itching, facial swelling, tightness of the chest, and wheezing [see Warnings and Precautions (5.1)].
- To contact their physician or treatment center for further treatment and/or assessment if they experience a lack of clinical response to factor VIII replacement therapy, as this may be a manifestation of an inhibitor [see Warnings and Precautions (5.2)].
- To contact their healthcare provider or go to the emergency department right away if a thromboembolic event should occur [see Warnings and Precautions (5.3)].
- That despite stringent procedures designed to reduce risk, the risk of transmitting infectious agents cannot be totally eliminated. Advise patients, especially pregnant women and immunocompromised individuals, to report any signs and symptoms of fever, rash, joint pain, or sore throat, to their physician immediately [see Warnings and Precautions (5.6)].
Manufactured by:
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U. S. License No. 1694
3063713
Principal Display Panel – 250 IU Vial Label
NDC 68516-4605-1 250 IU FVIII Range
Antihemophilic Factor/
von Willebrand Factor
Complex (Human)
Alphanate® 5 mL
Solvent Detergent / Heat Treated
Storage: Store at temperatures
not exceeding 25 °C (77 °F).
Rx only. Single dose container for
intravenous administration only.
GRIFOLS U.S. License No. 1694
Instructions: Reconstitute
with 5 mL of Sterile Water
for Injection, USP. Administer
promptly after reconstitution
and do not refrigerate. Discard
unused contents. For information
on dosage and directions for
administration, see accompanying
pamphlet. Contains no
preservatives. The patient and
physician should discuss the risks
and benefits of this product.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
Lot
EXP
IU FVIII/Vial
IU VWF:RCo/Vial 3063712
Lot
IU FVIII/Vial
IU VWF:RCo/Vial
Alphanate® 5 mL NDC 68516-4605-1

Principal Display Panel – 250 IU Carton Label
NDC 68516-4616-1 250 IU FVIII Range
Antihemophilic Factor/von Willebrand
Factor Complex (Human)
Alphanate®
Solvent Detergent / Heat Treated
Rx only
For Intravenous Administration 5 mL
GRIFOLS
Contents: One vial Antihemophilic Factor/von Willebrand
Factor Complex (Human), Alphanate®, one vial
5 mL Sterile Water for Injection, USP, one
Mix2Vial® filter transfer set, and directions for use.
Composition:
Factor VIII, von Willebrand Factor, Arginine, Albumin
and Histidine.
Contains no preservatives.
Administer within three hours of reconstitution.
Discard unused content.
GRIFOLS
Warning:
This product is prepared from large pools of
human plasma. Human blood and its components
may transmit infectious agents. See package
insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should
discuss the risks and benefits of this product.
For information on dosage and directions for
administration, see enclosed package insert.
Storage: Store at temperatures not exceeding
25 °C (77 °F). Do not freeze.
Single dose container for intravenous
administration only.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, USA
U.S. License No. 1694
GRIFOLS
3063711
GTIN 00368516461617
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXX XXXXXX
IU FVIII/Vial XXXX
IU VWF: RCo/Vial XXXX

Principal Display Panel – 500 IU Vial Label
NDC 68516-4606-1 500 IU FVIII Range
Antihemophilic Factor/
von Willebrand Factor
Complex (Human)
Alphanate® 5 mL
Solvent Detergent / Heat Treated
Storage: Store at temperatures
not exceeding 25 °C (77 °F).
Rx only. Single dose container for
intravenous administration only.
GRIFOLS U.S. License No. 1694
Instructions: Reconstitute
with 5 mL of Sterile Water
for Injection, USP. Administer
promptly after reconstitution
and do not refrigerate. Discard
unused contents. For information
on dosage and directions for
administration, see accompanying
pamphlet. Contains no
preservatives. The patient and
physician should discuss the risks
and benefits of this product.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
Lot
EXP
IU FVIII/Vial
IU VWF:RCo/Vial 3063710
Lot
IU FVIII/Vial
IU VWF:RCo/Vial
Alphanate® 5 mL NDC 68516-4606-1

Principal Display Panel – 500 IU Carton Label
NDC 68516-4617-1 500 IU FVIII Range
Antihemophilic Factor/von Willebrand
Factor Complex (Human)
Alphanate®
Solvent Detergent / Heat Treated
Rx only
For Intravenous Administration 5 mL
GRIFOLS
Contents:
One vial Antihemophilic Factor/von Willebrand
Factor Complex (Human), Alphanate®, one vial
5 mL Sterile Water for Injection, USP, one
Mix2Vial® filter transfer set, and directions for use.
Composition:
Factor VIII, von Willebrand Factor, Arginine, Albumin
and Histidine.
Contains no preservatives.
Administer within three hours of reconstitution.
Discard unused contents.
GRIFOLS
Warning:
This product is prepared from large pools of
human plasma. Human blood and its components
may transmit infectious agents. See package
insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should
discuss the risks and benefits of this product.
For information on dosage and directions for
administration, see enclosed package insert.
Storage: Store at temperatures not exceeding
25 °C (77 °F). Do not freeze.
Single dose container for intravenous
administration only.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, USA
U.S. License No. 1694
GRIFOLS
3063709
GTIN 00368516461716
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXX XXXXXX
IU FVIII/Vial XXXX
IU VWF: RCo/Vial XXXX

Principal Display Panel – 1000 IU Vial Label
NDC 68516-4607-2 1000 IU FVIII Range
Antihemophilic Factor/
von Willebrand Factor
Complex (Human)
Alphanate® 10 mL
Solvent Detergent / Heat Treated
Storage: Store at temperatures
not exceeding 25 °C (77 °F).
Rx only. Single dose container for
intravenous administration only.
GRIFOLS U.S. License No. 1694
Instructions: Reconstitute
with 10 mL of Sterile Water
for Injection, USP. Administer
promptly after reconstitution
and do not refrigerate. Discard
unused contents. For information
on dosage and directions for
administration, see accompanying
pamphlet. Contains no
preservatives. The patient and
physician should discuss the risks
and benefits of this product.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
Lot
EXP
IU FVIII/Vial
IU VWF:RCo/Vial 3063708
Lot
IU FVIII/Vial
IU VWF:RCo/Vial
Alphanate® 10 mL NDC 68516-4607-2

Principal Display Panel – 1000 IU Carton Label
NDC 68516-4618-2 1000 IU FVIII Range
Antihemophilic Factor/von Willebrand
Factor Complex (Human)
Alphanate®
Solvent Detergent / Heat Treated
Rx only
For Intravenous Administration 10 mL
GRIFOLS
Contents:
One vial Antihemophilic Factor/von Willebrand
Factor Complex (Human), Alphanate®, one vial
10 mL Sterile Water for Injection, USP, one
Mix2Vial® filter transfer set, and directions for use.
Composition:
Factor VIII, von Willebrand Factor, Arginine, Albumin
and Histidine.
Contains no preservatives.
Administer within three hours of reconstitution.
Discard unused contents.
GRIFOLS
Warning:
This product is prepared from large pools of
human plasma. Human blood and its components
may transmit infectious agents. See package
insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should
discuss the risks and benefits of this product.
For information on dosage and directions for
administration, see enclosed package insert.
Storage: Store at temperatures not exceeding
25 °C (77 °F). Do not freeze.
Single dose container for intravenous
administration only.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, USA
U.S. License No. 1694
GRIFOLS
3063707
GTIN 00368516461822
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXX XXXXXX
IU FVIII/Vial XXXX
IU VWF: RCo/Vial XXXX

Principal Display Panel – 1500 IU Vial Label
NDC 68516-4608-2 1500 IU FVIII Range
Antihemophilic Factor/
von Willebrand Factor
Complex (Human)
Alphanate® 10 mL
Solvent Detergent / Heat Treated
Storage: Store at temperatures
not exceeding 25 °C (77 °F).
Rx only. Single dose container for
intravenous administration only.
GRIFOLS U.S. License No. 1694
Instructions: Reconstitute
with 10 mL of Sterile Water
for Injection, USP. Administer
promptly after reconstitution
and do not refrigerate. Discard
unused contents. For information
on dosage and directions for
administration, see accompanying
pamphlet. Contains no
preservatives. The patient and
physician should discuss the risks
and benefits of this product.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
Lot
EXP
IU FVIII/Vial
IU VWF:RCo/Vial 3063706
Lot
IU FVIII/Vial
IU VWF:RCo/Vial
Alphanate®10 mL NDC 68516-4608-2

Principal Display Panel – 1500 IU Carton Label
NDC 68516-4619-2 1500 IU FVIII Range
Antihemophilic Factor/von Willebrand
Factor Complex (Human)
Alphanate®
Solvent Detergent / Heat Treated
Rx only
For Intravenous Administration 10 mL
GRIFOLS
Contents:
One vial Antihemophilic Factor/von Willebrand
Factor Complex (Human), Alphanate®, one vial
10 mL Sterile Water for Injection, USP, one
Mix2Vial® filter transfer set, and directions for use.
Composition:
Factor VIII, von Willebrand Factor, Arginine, Albumin
and Histidine.
Contains no preservatives.
Administer within three hours of reconstitution.
Discard unused contents.
GRIFOLS
Warning:
This product is prepared from large pools of
human plasma. Human blood and its components
may transmit infectious agents. See package
insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should
discuss the risks and benefits of this product.
For information on dosage and directions for
administration, see enclosed package insert.
Storage: Store at temperatures not exceeding
25 °C (77 °F). Do not freeze.
Single dose container for intravenous
administration only.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, USA
U.S. License No. 1694
GRIFOLS
3063705
GTIN 00368516461921
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXX XXXXXX
IU FVIII/Vial XXXX
IU VWF: RCo/Vial XXXX
Principal Display Panel – 2000 IU Vial Label
NDC 68516-4610-2 2000 IU FVIII Range
Antihemophilic Factor/
von Willebrand Factor
Complex (Human)
Alphanate® 10 mL
Solvent Detergent / Heat Treated
Storage: Store at temperatures
not exceeding 25 °C (77 °F).
Rx only. Single dose container for
intravenous administration only.
GRIFOLS U.S. License No. 1694
Instructions: Reconstitute
with 10 mL of Sterile Water
for Injection, USP. Administer
promptly after reconstitution
and do not refrigerate. Discard
unused contents. For information
on dosage and directions for
administration, see accompanying
pamphlet. Contains no
preservatives. The patient and
physician should discuss the risks
and benefits of this product.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
Lot
EXP
IU FVIII/Vial
IU VWF:RCo/Vial 3063704
Lot
10
IU FVIII/Vial
IU VWF:RCo/Vial
Alphanate®10 mL NDC 68516-4608-2

Principal Display Panel – 2000 IU Carton Label
NDC 68516-4620-2 2000 IU FVIII Range
Antihemophilic Factor/von Willebrand
Factor Complex (Human)
Alphanate®
Solvent Detergent / Heat Treated
Rx only
For Intravenous Administration 10 mL
GRIFOLS
Contents:
One vial Antihemophilic Factor/von Willebrand
Factor Complex (Human), Alphanate®, one vial
10 mL Sterile Water for Injection, USP, one
Mix2Vial® filter transfer set, and directions for use.
Composition:
Factor VIII, von Willebrand Factor, Arginine, Albumin
and Histidine.
Contains no preservatives.
Administer within three hours of reconstitution.
Discard unused contents.
GRIFOLS
Warning:
This product is prepared from large pools of
human plasma. Human blood and its components
may transmit infectious agents. See package
insert, WARNINGS AND PRECAUTIONS.
Instructions: The patient and physician should
discuss the risks and benefits of this product.
For information on dosage and directions for
administration, see enclosed package insert.
Storage: Store at temperatures not exceeding
25 °C (77 °F). Do not freeze.
Single dose container for intravenous
administration only.
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, USA
U.S. License No. 1694
GRIFOLS
3063703
GTIN 00368516462027
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXX XXXXXX
IU FVIII/Vial XXXX
IU VWF: RCo/Vial XXXX
Principal Display Panel – 5 mL Vial Label
NDC 76297-002-02
Sterile Water for Injection, USP
5 mL
Rx only
3057422
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other substance has been added. Do not use for intravascular injection without making approximately isotonic by addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S.A. Parets del Vallès,
Barcelona 08150 Spain
Lot
EXP

Principal Display Panel – 10 mL Vial Label
NDC 76297-002-12
Sterile Water for Injection, USP
10 mL
Rx only
3057423
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other substance has been added. Do not use for intravascular injection without making approximately isotonic by addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S.A. Parets del Vallès,
Barcelona 08150 Spain
Lot
EXP

Principal Display Panel – 5 mL Vial Label
NDC 68516-1001-1
Sterile Water for Injection, USP
5 mL
Rx only
3051532
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other substance has been added. Do not use for intravascular injection without making approximately isotonic by addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S.A. Parets del Vallès, Barcelona 08150 Spain
Mfd for: Grifols Biologicals LLC Los Angeles, CA 90032, USA
Lot
EXP

Principal Display Panel – 10 mL Vial Label
NDC 68516-1002-2
Sterile Water for Injection, USP
10 mL
Rx only
3051533
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other substance has been added. Do not use for intravascular injection without making approximately isotonic by addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S.A. Parets del Vallès, Barcelona 08150 Spain
Mfd for: Grifols Biologicals LLC Los Angeles, CA 90032, USA
Lot
EXP