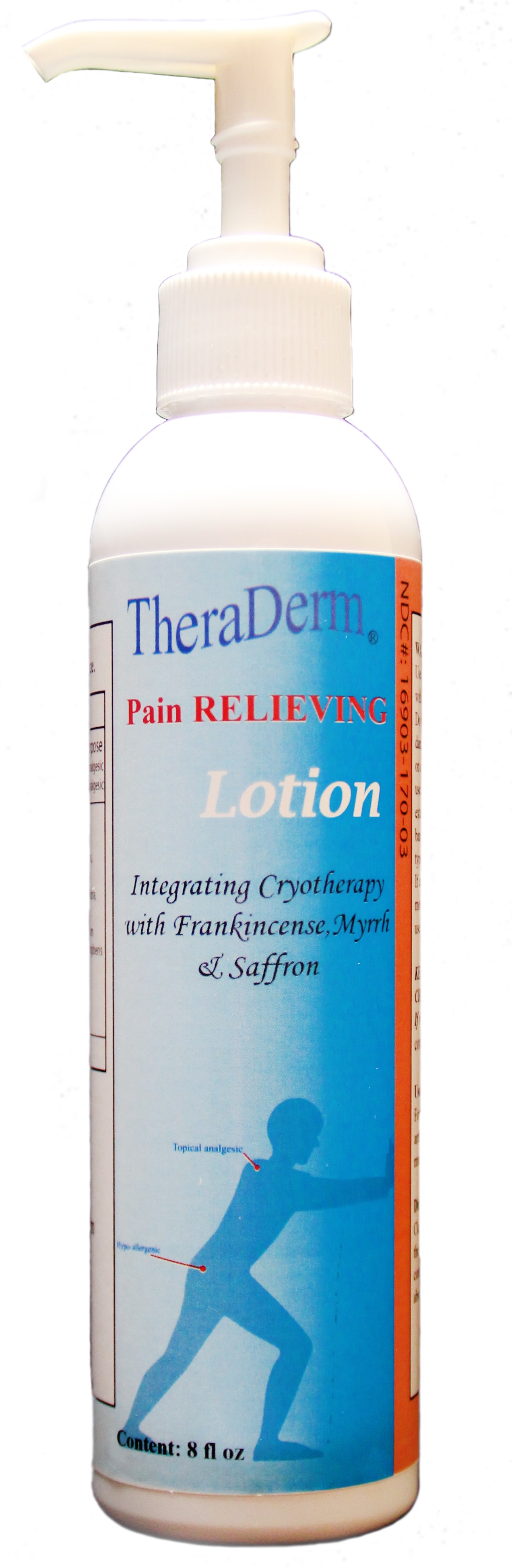
Directions:
For the temporary relief of minor arthritis pain, simple backache, muscle strains and sprains.
Clean affected Area and rub or massage area continuously until lotion has been absoprbed into the area.
Directions: clean the affected area, then apply to pain area. Rub or massage the area continuously until the lotion has been fully absorbed into the area.
Warning: for external use only
Use only as directed. Avoid contact with the eyes and mucous membranes. DO not apply to open wounds, damaged or very sensitive skin. Test on a small area before use. Do not use in combination with other external analgesic products. Do not bandage tightly or cover with any type of wrap, except clothing. If condition worsens, or persists for more than seven days, discontinue use and consult a physician.
KEEP OUT OF THE REACH OF CHILDREN
If swallowed, get medical help or contact Poison Control right away.
Inactive Ingredients
Salvia Miltiorrhiza root, Carthamus Tinctorius Flower Oil, Lavender Oil, Angelica Actiloba Flowering Top, Aloe,Zingiber Cassumunar Root Oil,Angelicae Pubescens Root,Stearic Acid, Prunus Persica Flower Bud,Notopterygium Franchetii Root,Frankincense Oil, Ligusticum Sinese Subsc, Chuanxiong Root, Myrrh,nGlycerin, Vitamin E Oil,Coconut Oil.
Purpose
The Purpose of our pain relieving Lotion is to provide temporary relief of minor aches and pains, arthritiis pain, backache, muscle aches and strains.