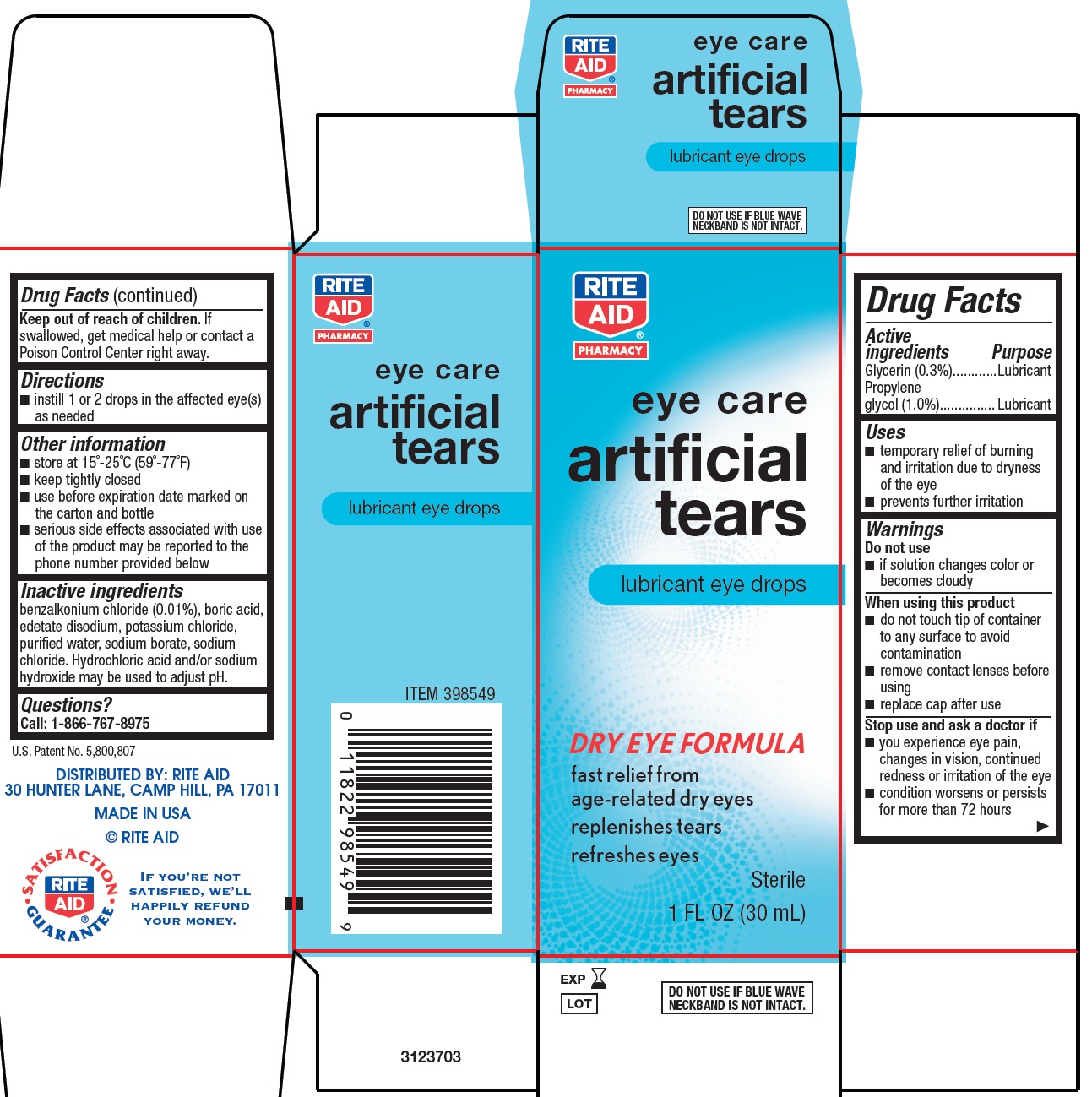
ARTIFICAL TEARS- glycerin and propylene glycol solution/ drops
Rite Aid Corporation
----------
Drug Facts
Uses
- •
- temporary relief of burning and irritation due to dryness of the eye
- •
- prevents further irritation
Warnings
When using this product
- •
- do not touch tip of container to any surface to avoid contamination
- •
- remove contact lenses before using
- •
- replace cap after use
Other information
- •
- store at 15°-25°C (59°-77°F)
- •
- keep tightly closed
- •
- use before expiration date marked on the carton and bottle
- •
- serious side effects associated with use of the product may be reported to the phone number provided below
Inactive ingredients
benzalkonium chloride (0.01%), boric acid, edetate disodium, potassium chloride, purified water, sodium borate, sodium chloride. Hydrochloric acid and/or sodium hydroxide may be used to adjust pH.
| ARTIFICAL TEARS
glycerin and propylene glycol solution/ drops |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Rite Aid Corporation (014578892) |
| Registrant - Baush & Lomb Incorporated (196603781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 114406598 | MANUFACTURE(11822-9854) | |
Revised: 12/2018
Document Id: 07056a34-8bd8-46db-9a8b-1ad597508774
Set id: 5a435dbc-669f-49c9-abf2-5e35f1e3d578
Version: 4
Effective Time: 20181203
Rite Aid Corporation