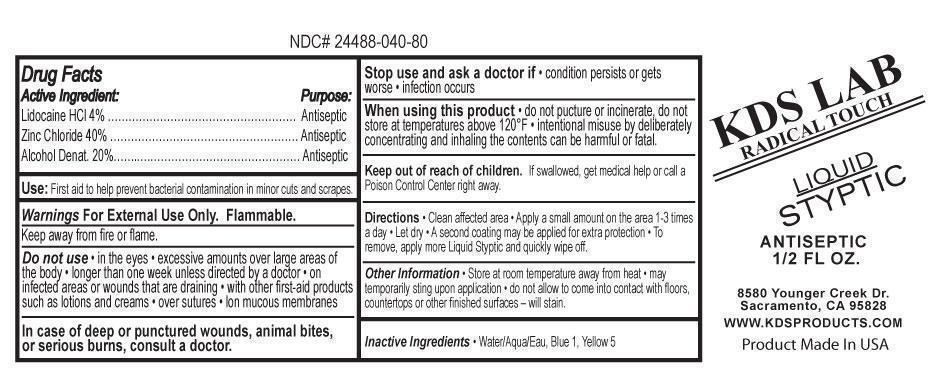
Active Ingredient:
Lidocaine HCl 4% ………………………………………………
Zinc Chloride 40% ………………………………………………
Alcohol Denat. 20%……..….……………………………………
Do not use
• in the eyes • excessive amounts over large areas of
the body • longer than one week unless directed by a doctor • on
infected areas or wounds that are draining • with other first-aid products
such as lotions and creams • over sutures • lon mucous membranes
In case of deep or punctured wounds, animal bites, or serious burns, consult a doctor.
In case of deep or punctured wounds, animal bites, or serious burns, consult a doctor.When using this product
• do not pucture or incinerate, do notstore at temperatures above 120°F • intentional misuse by deliberately
concentrating and inhaling the contents can be harmful or fatal.
Keep out of reach of children.
f swallowed, get medical help or call a
Poison Control Center right away.
Directions
Clean affected area • Apply a small amount on the area 1-3 timesa day • Let dry • A second coating may be applied for extra protection • To
remove, apply more Liquid Styptic and quickly wipe off.