METHIO-FORM- racemethionine tablet, chewable
LLOYD, Inc. of Iowa
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Methio-Form® Chewable Tablets
(DL-methionine)
Indications and Usage
For use as an aid in acidifying the urine of dogs and cats. Methio-Form is also an aid in controlling the odor from feline and canine urine residues.
Methio-Form Chewable Tablets may be used as a source of the essential amino acid, DL-methionine.
Description
Each scored Methio-Form Chewable Tablet contains 500 mg DL-methionine (6.7 milliequivalents [mEq]) in a palatable protein base.
Clinical Pharmacology
DL-methionine [2 amino-4-(methyl-thio)butyric acid], a sulfur-containing essential amino acid,1,2 is soluble in water and dilute acid, but is insoluble in most organic solvents. The pH of a 1% solution is 5.6 to 6.0. It has been established that methionine is the source of approximately 80% of the sulfur in cystine, one of the most important amino acids in mammalian metabolism3. The role of methionine in the formation of choline and creatinine is well known.3 Methionine ingested in excess of normal metabolic needs for the amino acid may be metabolized in many ways. A simplified diagram of the catabolism of methionine in the mammalian liver is presented below, and illustrates why DL-methionine may be an effective, continuous urinary acidifying agent4. One millimole of methionine (149.2 mg) as it is metabolized in the liver will theoretically produce 2 mEq of sulfuric acid, which is excreted largely in the urine.
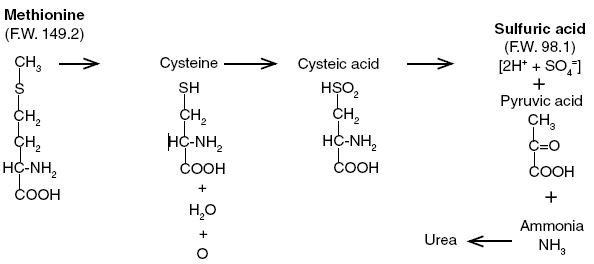
Methio-Form may be used to prevent the formation of magnesium ammonium phosphate hexahydrate (MgNH4PO4• 6H2O) crystals in the urine. These crystals are commonly called struvite. Struvite is the most frequently involved mineral in uroliths that occur in feline lower urinary tract disease (LUTD) also known as feline urologic syndrome (FUS). Struvite crystals are more soluble in acid urine and tend not to develop and will eventually dissolve as urinary pH is reduced.
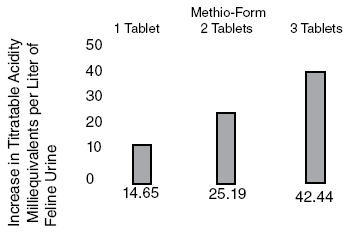
Methionine is effective in increasing titratable acidity6, a measure of buffering capacity on the ability to neutralize acids and bases. Methionine can be expected to decrease pH from control values (pH) approximately 0.80 pH units at a daily dose of 5 mEq(375mg)/kg body weight (Figure 1). This same dose has increased titratable acidity 42.4 mEq/liter of urine. (Figure 2).
Figure 1. Change in Feline urinary pH after administration of Methio-Form.
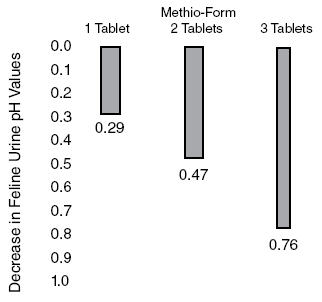
Figure 2. Change in Feline urine Titratable Acidity after administration of Methio-Form.
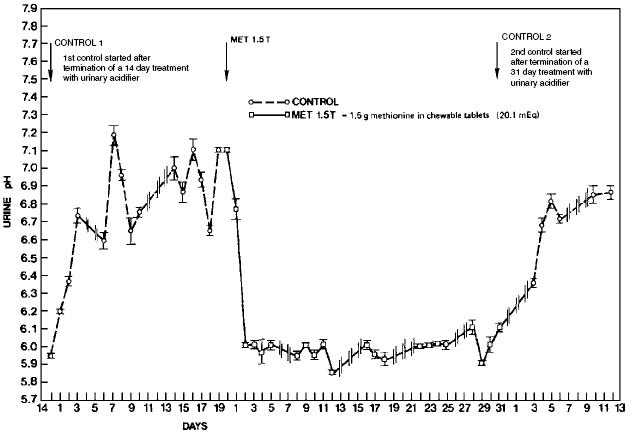
Urinary pH is primarily a function of diet, with proteins routinely decreasing pH and minerals generally increasing pH. The diets of cats and dogs may be expected to produce urine pH values of 6.6 to 7.2. Therefore, a dose of 5 mEq (375 mg) of methionine/kg body weight should effect a urinary pH value of 5.8 to 6.2. Daily administration is necessary to maintain continuous effects in pH and TA. Adaptation does not occur, but approximately 2 days of dosing is required to attain the maximum effects (Figure 3)6.
LUTD (FUS) is relatively common and affects both sexes; however, male cats are more frequently involved. Cystitis and urolithiasis produce post-renal uremia with elevated blood urea nitrogen (BUN), hyperphosphatemia, hyperkalemia, metabolic acidosis, and death if not treated.
The following principles have been suggested for uroliths in LUTD that are predominantly composed of struvite to reduce the concentration of pre-struvite crystalloids and formation of calculi:
- Reducing crystalloid concentration by reducing dietary concentration of magnesium and phosphorus to minimal requirements.
- Increasing urinary volume by increasing water intake.
- Increasing solubility of crystalloids by reducing urinary pH and holding at a reduced value7.
Methio-Form Chewable Tablets, are designed to furnish a convenient, effective and practical dosage form for urinary acidification. They reduce the client compliance problem of daily administration of conventional tablets. Methio-Form Chewable Tablets are highly palatable to cats and dogs.
Figure 3. Time-related effects of methionine on Feline urinary pH
Contraindications
Do not administer to animals with severe liver, kidney or pancreatic disease, or those which are acidotic due to conditions such as uncontrolled diabetes mellitus or urinary obstruction.
Warning
Methio-Form is extremely palatable to dogs and cats and overconsumption of tablets may result if access to open bottles occurs. The effect of overconsumption may be severe and could result in life-threatening metabolic acidosis if veterinary treatment is not initiated.
Also, excess consumption of methionine may result in the production of methyl mercaptan (methanethiol) which may be toxic. In animals with healthy livers, metabolites of methionine are converted to nontoxic substances. Generally, methionine in the diet at twice the required level is well tolerated, but at a threefold or above level toxicosis often results.
Precautions
In rare cases animals may experience gastrointestinal disturbance. In those cases, administer during feeding or in two to three divided doses.
Caution should be advised against overdosing animals to avoid chronic over acidification which could lead to potential problems such as acidosis, potassium wasting, osteoporosis, Heinz body formation, and possible precipitation of non-struvite minerals in the urine.
Cats have been frequently poisoned by ingesting numerous tablets from bottles which were left open. The intoxication resulting from accidental over consumption is primarily an acidemia. Cats which are presented for treatment within 4 hours after a substantial over consumption event will benefit from emptying the stomach by use of emetics or gavage. Activated charcoal (ToxiBan™, Vet-A-Mix) will be helpful if administered in oral doses of one to two g/kg body weight with or without gavage and before cats become severely intoxicated with signs of ataxia, depression and coma. The recommended treatments for the acidosis include parenteral solutions, such as saline-bicarbonate with dextrose, lactated Ringer, lactate, bicarbonate, or Darrow's or Butler's for acidosis. Sodium bicarbonate administered orally may be helpful unless the animal has been vomiting frequently. Avoid the use of ammonium salts of solutions. Adult cats ingesting 20 Methio-Form tablets at one time may be expected to become nonlethally intoxicated, but cats ingesting 40 or more tablets will probably die without treatment.
Administration
Methio-Form Chewable Tablets may be fed free choice, from the hand or may be crumbled and mixed into the food. Dosing one time each day is appropriate, but daily doses may be administered in 2 or 3 divided doses if more convenient or in rare cases where animals may vomit a single daily dose.
For urinary acidification in cats and dogs and prevention of struvite urolithiasis urinary pH should be reduced to approximately 6.0 - 6.6 and maintained in this range. Monitoring the pH of freshly voided urine may be advisable.
Dosage
Daily dosages vary with diets and amount of acidification needed. After dosages have been determined, they may be used continuously.
Cats
The usual daily dose is 2.5 to 5.0 mEq (188-375 mg)/kg body weight or ½ to one tablet per 1 to 1.5 kg. (2.5 to 3 lb) body weight. Average size adult cats normally should receive 1½ to 3 tablets (10-20 mEq) daily.
Dogs
The usual daily dose is 2 to 4 mEq (150-300 mg)/kg body weight. Small breeds - 7 kg (15 lb) or under, ½ to 4 tablets. Medium Breeds - 7 to 15 kg (15-33 lb), 2 to 7 tablets. Large Breeds - 15-30 kg (33-66 lb), 4 to 13 tablets.
Note: Methio-Form tablets can be crumbled and sprinkled on the animal's food. A level teaspoonful of a crumbled tablet contains 746 mg (10.0 mEq) of DL-methionine.
How Supplied
| 50 tablet bottles | List No. 2403 |
| 150 tablet bottles | List No. 2404 |
| 500 tablet bottles | List No. 2402 |
Patent Pending
Bibliography
- Nutrient Requirements of Cats, Rev. 1986, National Research Council, National Academy of Sciences, page 11.
- Nutrient Requirements of Dogs, Rev. 1985, National Research Council, National Academy of Sciences, page 11.
- Osol, Arthur and Farrar, George: The Dispensatory of the United States of America, 25th ed., 1960, J. B. Lippincott Co., Philadelphia, PA, page 850.
- Bell, George; Davidson, J., and Scarborough, Harold: Textbook of Physiology and Biochemistry, ed. 6, 1965, The Williams and Wilkins Company, Baltimore, MD, pages 364 and 365.
- Goodman, L. and Gilman, A: The Pharmacological Basis of Therapeutics, ed. 8, 1980, Pergamon Press, NY, page 697.
- Lloyd, WE; Sullivan, DJ: Effects of orally administered ammonium chloride and methionine on feline urinary acidity, VM/SAC 79: 773-778, 1984.
- Osborne, CA; Lees, GE: Feline Cystitis, Urethritis, Urethral Obstruction Syndrome, Mod Vet Pract59:513-518, 1978.
| METHIO-FORM
racemethionine tablet, chewable |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - LLOYD, Inc. of Iowa (962286535) |
| Registrant - LLOYD, Inc. of Iowa (007281942) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LLOYD, Inc. of Iowa | 962286535 | LABEL, API MANUFACTURE, MANUFACTURE, PACK | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LLOYD, Inc. of Iowa | 007281942 | ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sumitomo Chemical Company, Limited | 711530345 | API MANUFACTURE | |
