Warning
1. ESTROGENS HAVE BEEN REPORTED TO INCREASE THE RISK OF ENDOMETRIAL CARCINOMA.
Three independent case control studies have shown an increased risk od endometrial cancer in postmenopausal women exposed to exogenous estrogens for prolonged periods. 1-3 The risk was independent of other known risk factors for endometrial cancer. These studies are further supported by the finding that incidence rates of endometrial cancer have increased sharply since 1969 in eight different areas of the United States with population-based cancer reporting systems, an increase which may be related to rapidly expanding use of estrogens during the last decade. 4
The three case control studies reported that the riskof endometrial cancer in estrogen users was about 4.5 to 13.9 times greater than in nonusers. The rik appears to depend on both duration of treatment 1 and on estrogen dose. 3 In view of these findings, when estrogens are used for the treatment of menopausalsymptoms, the lowest dose that will controlsymptoms should be utilized and medication should be discontinuedas soon as possible. When prolonged treatment is medically indicated, the patient should be reassessedon at least a semiannual basis to determine the need for continued therapy.
Although the evidence must be considered preliminary, one study suggests that cyclic administration in low doses of estrogen may carry less risk than continuos administration, 3 it therefore appears prudent to utilize such a regimen.
Close clinical surveillance of all women taking estrogens is important. In all cases of undiagnosed persistant or recurring abnormal vaginal bleeding, adequate diagnostic measures should be undertaken to rule out malignancy.
There is no evidence at present that "natural" estrogens are more or less hazardous than "synthetic" estrogens at equiestrogenic doses.
2. ESTROGENS SHOULD NOT BE USED DURING PREGNANCY The use of female sex hormones, both estrogens and progestens, during early pregnancy may seriously damage the offspring. It has been shown that females exposed in utero to diethylstilbestrol, a non-steroidal estrogen, have increased risk of developing in later life a form of vaginal or cervical cancer that is ordinarily extremely rare. 5,6 This risk has been estimated as not greater than 4 per 1000 exposures. 7 Furthermore, a highpercentage of such exposed women (from 30 to 90 percent) have been found to have vaginal adenosis, 8-12 epithelial changes of the vagina and cervix. Although these changes are histologically benign, it is not known whether they are precursors of malignancy. Although similar dataare not availablewith the use of other estrogens, it cannot be presumed they would not induce similar changes.
Several reports suggest an association between intrauterine exposure to female sex hormones and congenital anomalies, including congenital heart defects and limb reduction defects. 13-16 One case control study 16 estimated a 4.7 fold increased risk of limb reduction defects in infants exposed in utero to sex hormones (oral contraceptives, hormones withdrawl tests for pregnancy, or attempted treatment for threatened abortion). Some of these exposures were very short and involved only a few days of treatment. The data suggest that the risk of limb reduction defects in exposed fetuses is somewhat less than 1 per 1000.
In the past, female sex hormones have been used during pregnancy in an attempt to treat threatened or habitual abortion. There is no considerable evidence from well controlled studies that progestens are effective for these uses.
If Estrapel is used during pregnancy, or if the patient becomes pregnant while taking this drug, she should be apprised of the potential risks to the fetus, and the advisability of pregnancy continuation.
DESCRIPTION:
Estradiol N.F. Pellets contain N.F. 25mg and 50mg for subcutaneous implantation. The pellets are sterile unless vial has been opened or damaged.
Estradiol Pellets are cylindrical, with an approximate diameter of 3.2mm. Pellet therapy has the advantage of high efficiency from the standpoint of the quality of the hormone administered and the further advantage that a single treatment has effect continuously for several months.
CLINICAL PHARMACOLOGY:
Estradiol is one of the more potent of the known estrogenic compounds, identical with the primary estrogenic hormone produced by the human ovary. Estradiol exerts a developmental action on the female generative tract, has an inhibitory effect upon the pituitary in large doses, and produces a marked constitutional effect with an increase in muscular strength, body vigor, and mental acumen. It supplies follicular hormone in cases where estrogenic activity is depressed, insufficient, or absent.
INDICATIONS:
Estradiol is indicated in the treatment of:
1. Estrogen deficiency in Hysterectomized Women.
(There is no evidence that estrogens are effective for nervous symptoms or depression which might occur during menopause, and they should not be used to treat these conditions.)
2. Atrophic vaginitis.
3. Kraurosis vulvae.
4. Female hypogonadism.
5. Female castration.
6. Primary ovarian failure.
7. Breast cancer (for palliation only) in approximately selected women and men with metastatic disease.
8. Postpartum breast engorgement- Althoughestrogens have been widely used for the prevention of postpatum breast engorgement, controlled studies hve demonstrated that the incidence of significapainful engorgementin patients not receiving such hormonal therapy is low and usually responsive to appropriate analgesic or other supportive therapy. Consequently, the benefit to be derived from estrogen therapy for this indication must be carefully weighed against the potential increased risk or puerperal thromboembolism associated with the use of large doses of estrogen. 20,22
ESTRADIOL HAS NOT BEEN SHOWN TO BE EFFECTIVE FOR ANY PURPOSE DURIG PREGNANCY AND ITS USE MAY CAUSE SEVERE HARM TO THE FETUS (SEE BOXED WARNING).
CONTRAINDICATIONS:
Estrogens should not be used in women (or men) with any of the following conditions:
1. Known or suspected cancer of the breast except in appropriately selected patients being treated for metastatic disease.
2. Known or suspected estrogen-dependant neoplasia.
3. Known or suspected pregnancy (See Boxed Warning).
4. Undiagnosed abnormal genital bleeding.
5. Active thrombophlebitis or thromboembolic disorders.
6. Apast history of thrombophlebitis, thrombosis, or thromboembolic disorders associated with previous estrogen use (except when used in treatment of breast prostatic malignancy).
WARNINGS:
1. Induction of malignant neoplasma.nLong term continuous administration of natural and synthetic estrogen in certain animal species increases the frequency of carcinomas of the breast, cervix, vagina, and liver. There is new evidence that estrogens increase the risk of carcinoma of the endometrium in humans. (See Boxed Warning.)
At the present ime there is no satisfactory evidencethat estrogens given to postmenopausal women increase the risk of cancer of the breast, 18 althogh a recent long-term followup of a single physician's practice has raised this possibility. 18a Because of the animal data there is a heed for caution, in prescribing estrogens for women with a strong history of breast cancer or who have breast nodules, fibrocystic disease, or abnormal mammograms.
2. Gallbladder disease. A recent study has reported a 2 to 3 -fold increase in the risk of surgically confirmed gall bladder disease in women receiving postmenopausal estrogens 18 similar to the 2 fold increase previously noted in users of oral contraceptives. 19,24 In the case of oral contraceptives the increased risk appeared after two years of use. 24
3. Effects similar to those caused by estrogen-progsten oral contraceptives. There are several serios adverse effects or oral contraceptives, most of which have not, up to now, benn documented as consequences of postmenopausal estrogen therapy. This may reflect the comparatively low doses of estrogen used in postmenopausal women. It would be expected that the larger doses of estrogen used to treat prostatic or breast cancer or postpartm brast engorgement are more likely to result in these adverse effects, and, in fact,it has been shown that there is an increased risk of thrombosis in men receiving estrogens for prostatic cancer and women for postpartum breast engorgement.
a. Thromboembolic disease. It is now we establish that users of oral contraceptives have an increased risk of various thromboembolicand thrombotic vascular disease, such as thrombophlebitis, pulminary embolism, stroke, and myocardial infarction. 24-31 Cases of retinal thrombosis, mesenteric thrombosis and optic neuritishave been reported in oral contraceptive users. There is evidence that the risk of several of thse adverse reactions is related to the dose of the drug. 32,33 An increase risk of postsurgerythromboembolic complications has also been reported in users of oral contrceptives. 34,35 if feasable, estrogen should be discontinued at least 4 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
While an increased rate of thromboembolic and thrombotic disease in postmenopausal users of estrogen has not been found, 18,36 this does not rule out that such an increase maymay be present or that sugroups of women having underlyingrisk factors or who are receiving relatively large doses of estrogen may have an increased risk. Therefore estrogens should not be used in persons with active thrombophlebitis or thromboembolic disorders and they should not be used (except in treatent of malignancy) in persons with a history of such disorders in association with estrogen use. They should be used with caution in patients with cerebral vascular or coronary artery disease and only for those in whom estrogens are clearly needed.
Large doses of estrogens (5mg conjugated estrogens per day), comparable to those used to treat cancer of the prostat and breast, have been shown in a large prospectiveclinical trial in men 37 to increase the risk of nonfatal myocardial infarction, pulmonary embolism, and thrombophlebitis. When estrogen doses of this size are used, any of th thromboembolicand thrombotic adverse effects associated with oral contraceptive use should be considered a clear risk.
b. Hepatic adenoma. Benign hepatic adenomas appear to be associated with the use of oral contraceptives. 38-40 Although benign, and rare, these may rupture and may cause death through intraabdominal hemorrhage. Such lesions have not yet been reported in association with other estrogenor progesten preparations but should be considered in estrogen users having abdominalpain and tenderness,abdominal mass, or hypovolemic shock. Hepatocellular carcinoma has also been reported in women taking estrogen-containing orarl contraceptives. 39 The relationship of this malignancy to these drugs is not know at this time.
c. Elevated blood pressure. Increased blood pressure is not uncommon in women using oral contraceptives. There is now a report that this may occur with the use of estrogens in menopause 41 and blood pressure should be monitoredwith estrogen use, especially if high doses are used.
d. Glucose tolerance. A worsening of glucose tolerance has been observed in a significant percentage of patients on estrogen-containing oral contraceptives. For this reason, diabetic patients should be carefully observed while receiving estrogen.
4. Hypercalcemia. Administration of estrogens may lead to severe hypercalceemia in patients with breast cancer and bone metastases. If this occurs, the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
PRECAUTIONS:
A. General Precautions.
1. A complete medical and family history should be taken prior to initiation of any estrogen therapy. The pretreatment and periodic physical examination should include special reference to blood pressure, breasts, abdomen, and pelvic organs, and should include a Papnicolau smear. As a general rule, estrogen should not be prescribed for longer than one year without a physical examinationbeing performed.
2. Fluid retention- Because estrogens may cause some degree of fluid retention, conditions which may be influenced by this factor such as epilepsy, migraine, and cardiac or renal dysfunction, require careful observation.
3. Certain patients may develope undesirable manifestations of excessive estrogenic stimulation, such as abnormal or excessive uterine bleeding, mastodynia, etc.
4.Oral contraceptives appear to be associated with an increased incidence of mental depression. 24 Although it is not clear whether this is due to the estrogenic or progestogenic component of the contraceptive, patients with a history of depression should be carefully observed.
5. Preexisting uterine leiomyomata may increase in size during estrogen use.
6. The pathologist should be advised of estrogen therapy when relevent specimens are submitted.
7. Patients with a history of jaundice during pregnancy have an increased risk of reccuranceof jaundice while receiving estrogen-containing oral contraceptive therapy. If jaundice develops in any patient receiving estrogen, the medication should be discontinued while the cause is investigated.
8. Estrogens may be poorly metabolized in patients with impaired liver function and they should be administered with caution in such patients.
9. Because estrogens influence the metabolism of calcium and phosphorous, they should be used with caution in patients with metabolic bone diseases that are associated with hypercalcemia or in patients with renal insufficiency.
10. Because of the effects of estrogens on epiphyseal closure, they should be used judiciously in young patients in whom bone growth is not complete.
11. Certain endocrine and liver function tests may be affected by estrogen-containing oral contraceptives. The following similar changes may be expected with larger doses of estrogen;
a. Increased sullobromophthalein retention.
b. Increased prothrombin and factors VII, VIII, IX, and X; decreased antithrombin 3; increased norepinephrine-induced platelet aggregability.
c. Increased thyroid binding globulin (TGB) leading to increased circulating total thyroid hormone, as measured by PBI, T4 by column, or T4 by radioimmunoassay. Free T3 resin uptake is decreased, reflecting elevated TBG; free T4 concentration is unaltered.
d. Impaired glucose toerance.
e. Decreased pregnanediol excretion.
f. Reduced response to metyrapone test.
g. Reduced serum folate concentration.
h. Increased serum triglyceride and phospholipid concentration.
B. Pregnancy Category X
(See Contraindications and Boxed Warning).
C. Nursing Mothers.
As a general principal, the administration of any drug to nursing mothers should be done only when clearly necessary since many drugs are excreted in human milk.
ADVERSE REACTIONS:
(See Warnings regarding induction of neoplasia, adverse effects on the fetus, increased incidence of gall bladder disease, and adverse effects similar to those of oral contraceptives, including thromboembolism.) The following additional adverse reactions have been reported with estrogenic therapy, including oral cantraceptives:
1. Genitourinary System- breakthrough bleeding, spotting, change in menstrual flow;dysmenorrhea; premenstrual-like syndrome; amenorrhea during and after treatment; increase in size of uterine fibromyomata; vaginal candidas; change in cervical eversion and in degree of cervical secretion; cystitis-like syndrome.
2. Breasts- tenderness, enlargement, secretion
3. Gastrointestinal- nausea, vomiting,; abdominal cramps, bloating; cholestatic jaundice.
4. Skin- chloasma or melasma which may persist when drug is discontinued; erythema multiforme; erythema nodosum; hemorrhagic eruption; loss of scalp hair; hirsutism.
5. Eyes- steepening of corneal curvature; intolerance to contact lenses.
6. CNS- headache, migraine, dizziness, mental depression, chorea.
7. Miscellaneous- increase or decrease in weight;reduced carbohydrate tolerance; aggrevation of porphyria; edema, changes in libido.
ACUTE OVERDOSAGE:
Numerous reports of ingestion of large doses of estrogen-containing oral contraceptives by young children indicate that serious ill effects do not occur. Overdosage of estrogen may cause nausea, and withdrawl bleeding may occur in females.
DOSAGE AND ADMINISTRATION:
Menopausal Syndrome: In all cases the objective should be determination of the minimum amount of hormone that will maintain the patient symptom-free. With adequate clinical improvement, usually obtainable in two weeks or less, gradual reduction in dosage are advisable. Subcutaneous implantation- implant one 25mg, Estradiol Pellet and repeat when necessary. The pellets provide constant estrogen levels for approximately 3 months.
Hypogenitalism and Sexual Infantilism: -1.5mg of estradiol or 1.66mg of estradiol benzoate intramuscularly two to three times weekly. Subcutaneous implantation -implant one 25mg, Pellet and repeat when necessary.
Amenorrhea and Oligomenorrhea Associated with Hypogonadism: 1.5mg of estradiol or 1.66mg of estradiol benzoate intramuscularly two to three times weekly during the first two weeks of an arbitrary 28-day menstrual cycle; progesterone is given the last two weeks of the theoretical cycle. This regimen is continued for 3-6 months. The patient then is allowed to go untreated for 2 months to determine whether or not she can maintain the cycle without hormonal therapy. If not, additional courses of therapy as outlined should be prescribed.
Postpartum Breast Engorgement: -1.5mg of estradiol or 1.66mg of estradiol benzoate is administered intramuscularlydaily begining at the first sign of engorgement and continuing until the symptoms are controlled. Restrictionof fluids and a tight binder should also be employed.
Inoperable Breast Carcinoma in Postmenopausal Women: -1.5mg of estradiol or 1.66mg of estradiol benzoate intramuscularly three or more times weekly according to the severity of the pain.
Carcinoma of the Prostate: -1.5mg of estradiol or 1.66mg of estradiol benzoate intramuscularly three times weekly. Subcutaneous Implantation- Implant one 25mg pellet and repeat when necessary.
Senile Vaginitis; Pruritis Vulvae; Kraurosis Vulvae: -1.0 to 1.5mg of estradiol or 1.0 to 1.66mg of estradiol benzoate intramuscularly three times weekly for two or three injections, then 0.5 to 1.0mg of estradiol or 0.33 to 1.0mg of estradiol benzoate twice weekly for maintainance.
The pellets may be implanted conveniently and quickly by means of an injector or they may be administered by making an incision in the skin. Either method, though readily carried out in the physician's office, is a minor surgical procedure, and all aseptic precautions must be observed.
BY INJECTOR: The pellet may be quickly and easily implanted by means of the Bardani or Bartor Pellet Injectors. The areas usually selected for implantation are the intrascapular region or the posterior axillary line. Aseptic precautions must be observed for any surgical procedure. The skin is carefully cleaned, followed by the application of iodine and alcohol. The area is infiltrated with procaine 1:100. Make a very small incision (about 2mm long and 1mm deep) into the skinwith a sharp scalpel to allow free passage of the large injector needle. The injector needle of the Kearns injector, with sharp plunger in place, is inserted into the incision and gently forced into the subcutaneous tissue at the desired site of implantation. The sharp plunger is withdrawn, and the pellet inserted into the hollow needle. The simplest method for placing the pellet in the needle is to allow the pellet to slide from the vial in which it is packed into the slot provided in the needle. The pellet is pushed as far as possible through the needle by means of the blunt plunger and held in place with the plunger while the needle is gently withdrawn. When the needle comes in contact with the knob of the plunger, both are withdrawn together. When the injector has been withdrawn, the wound may be closed with a single stitch or a skin clip. In many instances, apposition of the edges of the wound with adhesive tape is sufficient.
BY INCISION: The intrascapular region or the posterior axillary line are convenient site for implanting pellets. The operative field is prepared in the usual manner with iodine and alcohol and the area is infiltrated with procaine 1:100 solution. An incision about 1 centimeter in length is made. With blunt dissection, a pocket about two centimeters in depth is prepared in the subcutaneous tissue below and away from the incision. The edges of the pocket may be held apart by a smal dilator and the pellet inserted into the bottom of the pocket with small forceps. Force should not be used when inserting pellets. The incision is closed with one or two sutures.
Treated patients with an intact uterus should be monitored closely for signs of endometrial cancer and appropriate diagnostic measures should be taken to rule out malignancy in the event of persistent or recurring abnormal vaginal bleeding.
PACKAGING:
25mg:
Vial of one 25mg, pellet, box of 3; 10; 100
50mg:
Vial of one 50mg, pellet, box of 3; 10; 100
References:
1. Zeil, H.K. and W.D. Finkle, Increased Risk of Endometrial Carcinoma Among Users of Conjugated Estrogens, New England Journal of Medicine 293:1167-1170, 1975.
2. Smith, D.C., R. Prentic, D.J. Thompson, and W.L. Hermann, Association of Exogenous Estrogen and Endometrial Carcinoma, New England Journal of Medicine 293:1164-1167, 1975
3. Mack, T.M., M.C. Pike, B.E. Henderson, R.I. Pfeffer, V.R. Gerkins, M. Arthur, and S.E. Brown, Estrogens and Endometrial Cancer in a Retirement Community, New England Journal of Medicine 294:1262-1267, 1976.
4. Weiss, N.S., D.R. Szekely and D.F. Austin, Increasing Incidence of Endometrial Cancer in the United States, New England Journal of Medicine 294:1259-1262, 1976.
5. Herbst, A.L., H. Ulfelder and D.C. Poskanzer, Adenocarcinoma of Vagina, New England Journal of Medicine 248:878-881, 1971.
6. Greenwald, P., J. Barlow, P. Nasca, and W. Burnett, Vaginal Cancer after Maternal Treatment with Synthetic Estrogens, New England Journal of Medicine 285:390-392, 1971.
7. Lanier, A., K. Noller, D. Decker, L. Elveback, and L. Kurland, Cancer and Stilbestrol. A Follow-up of 1719 Persons Exposed to Estrogens In Utero and Born 1943-1959, Mayo Clinic Proceedings 48:793- 799, 1973.
8. Herbst, A., R. Kurman, and R. Scully, Vaginal and Cervical Abnormalities After Exposure to Stilbestrol ln Utero, Obstetrics and Gynecology 40:287-298, 1972.
9. Herbst, A., S. Robboy, G. Macdonald, and R. Scully, The Effects of Local Progesterone on Stilbestrol- Associated Vaginal Adenosis, American Journal of Obstetrics and Gynecology 118:607-615, 1974.
10. Herbst, A., D. Poskanzer, S. Robboy, L. Friedlander, and R. Scully, Prenatal Exposure to Stilbestrol A Prospective Comparison of Exposed Female Offspring with Unexposed Controls, New England Journal of Medicine 292:334-339, 1975.
11. Stall, A., R. Mattingly, D. Foley, and W. Fetherston, Clinical Diagnosis of Vaginal Adenosis, Obstetrics and Gynecology 43:118-128, 1974.
12. Sherman, A.l., M. Goldrath, A. Berlin, V. Vakhariya, F. Banooni, W. Michaels, P. Goodman, S. Brown, Cervical-Vaginal Adenosis After In Utero Exposure to Synthetic Estrogens, Obstetrics and Gynecology 44:531-545, 1974.
13. Gal, I., B. Kirman, and J. Stern, Hormone Pregnancy Tests and Congenital Maltormation, Nature 216:83, 1967.
14. Levy, E.P., A. Cohen, and F.C. Fraser, Hormone Treatment During Pregnancy and Congenital Heart Defects, Lancet 1:611, 1973. 15. Nora, J. and A. Nora, Birth Detects and Oral Contraceptives, Lancet 1:1941-942, 1973.
16. Janerich, D.T., J.M. Piper, and D.M. Glebatitis, Oral Centraoeptives and Congential Limb-Reduction Defects, New England Journal of Medicine 291:697-700, 1974.
17. Estrogens for Oral or Parenteral Use, Federal Register 40:8212, 1975.
18. Boston Collaborative Drug Surveillance Program Surgically Con rmed Gall Bladder Disease, Venous Thromboembolism and Breast Tumors in Relation to Post-Menopausal Estrogen Therapy, New England Journal of Medicine 290:15-19, 1974.
18a.Hoover, R., L.A. Gray, Sr., P. Cole, and B. MacMahon, Menopausal Estrogens and Breast Cancer, New England Journal of Medicine 295:401-405, 1976.
19. Boston Collaborative Drug Surveillance Program, Oral Contraceptives and Venous Thromboembclic Disease, Surgically Confirmed Gall Bladder Disease, and Breast Tumors, Lancet 1:1399-1404, 1973.
20. Daniel, D.G., H. Campbell, and A.C. Turnbull, Puerperal Thromboembolism and Suppression of Lactation, Lancet 2:287-289, 1967.
21. The Veterans Administration Cooperative Urological Research Group, Carcinoma of the Prostate: Treatment Comparisons, Journal of Urology 98:516-522, 1967.
22. Bailar, J.C., Thromboembolism and Oestrogen Therapy, Lancet 2:560, 1967.
23. Blackard, C., R. Doe, G. Mellinger, and D. Byar, Incidence of Cardiovascular Disease and Death in Patients Receiving Diethylstilbestrol for Carcinoma of the Prostate, Cancer 26:249-256, 1970.
24. Royal College of General Practitioners, Oral Contraception and Thromboembolic Disease, Journal of the Royal College of General Practitioners 13:267-279, 1967.
25. Inman, W.H. W. and M.P. Vessey, Investigation of Deaths from Pulmonary, Coronary, and Cerebral Thrombosis and Embolism in Women of Child-Bearing Age, British Medical Journal 2:193-199, 1968.
26. Vessey, M.P. and R. Doll, Investigation of Relation Between Use of Oral Contraceptlves and Thromboembolic Disease. A Further Report, British Medical Journal 2:651-657, 1969.
27. Sartweil, P.E., A.T. Masi, F.G. Arthes, G.R. Greene, and H.E. Smith, Thromboembolism and Oral Contraceptives: An Epidemiological Case Control Study, American Journal of Epidemiology 90:365- 380, 1969.
28. Collaborative Group for the Study of Stroke in Young Women, Oral Contraception and Increased Risk of Cerebral ischemia or Thrombosis, New England Journal of Medicine 288:871-878, 1973.
29. Collaborative Group for the Study of Stroke in Young Women, Oral Contraceptives and Stroke in Young Women: Associated Risk Factors, Journal of the American Medical Association 231:718-722. 1975.
30. Mann, J.l. and W.H.W. Inman, Oral Contraceptives and Death from Myocardial Infarction, British Medical Journal 2:245-248, 1975.
31. Mann, J.|., M.P. Vessey, M. Thorogood, and R. Doll, Myocardial lnfarction in Young Women with Special Reference to Oral Contraceptive Practice, British Medical Journal 2:241-245, 1975.
32. Inman, W,H.W., V.P. Vessey, B. Westerholm, and A. Engelund, Thromboembolic Disease and the Steroidal Content of Oral Contraceptives, British Medical Journal 2:203-209, 1970.
33.Stolley, P.D., J.A.Tonascia, M.S.Tockman, P.E.Sartwell, A.H.Rutledge, and M.P, Jacobs.Thrombosis with Low-Estrogen Oral Contraceptives, American Journal of Epidemiology 102:197-208, 1975.
34. Vessey, M.P., R. Doll, A.S. Fairbairn and G. Glober, Post-Operative Thromboembolism and the Use of the Oral Contraceptives, British Medical Journal 3:123-126, 1970.
35. Greene, G.R. and P.E. Sartwell, Oral Contraceptive Use in Patients with Thromboembolism Following Surgery, Trauma or lnfection, American Journal of Public Health 62:680-685, 1972.
36. Rosenberg, L., M.B. Armstrong and H. Jick, Myocardial Infarction and Estrogen Therapy in Postmenopausal Women, New England Journal of Medicine 294:1256-1259,1976.
37. Coronary Drug Project Research Group, The Coronary Drug Project: initial Findings Leading to Modi cations of Its Research Protocol, Journal of the American Medical Association 214:1303-1313, 1970.
38. Baum, J., F, Holtz, J.J. Bookstein, and E.W. Klein, Possible Association Between Benign Hepatomas and Oral Contraceptives, Lancet 2:926-928. 1973.
39. Mays, E.T., W.M. Christopherson, M.M. Mahr, and H.C. Williams, Hepatic Changes in Young Women Ingesting Contraceptive Steroids, Hepatic Hemorrhage and Primary Hepatic Tumors, Journal of the American Medical Association 235:730-782, 1976.
40. Edmondson, H.A., B. Henderson, and B. Benton, Liver Cell Adenomas Associated with the Use of Oral Contraceptives, New England Journal of Medicine 294:470-472, 1976.
41. Pteiter, Rl. and S. Van Den Noort, Estrogen Use and Stroke Risk in Postmenopausal Women, American Journal of Epidemiology 103:445-456, 1976.
ADVANCED PHARMACEUTICAL TECHNOLOGY
132 South Central Avenue, Elmsford, NY 10523
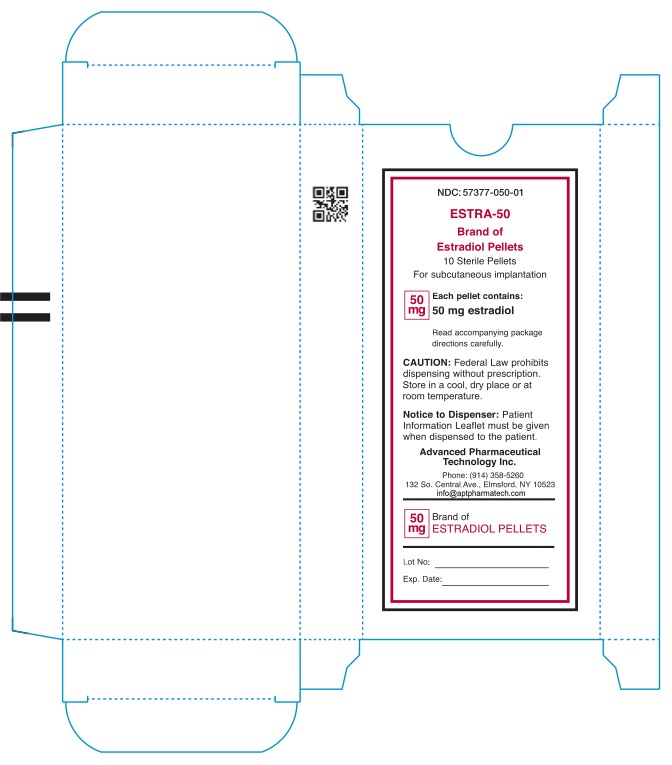
NDC: 57377-050-01
ESTRA-50
Brand of Estradiol Pellets
10 Sterile Pellets
For subcutaneous implantation
50mg
Each pellet contains:
50mg estradiol
Read accompanying package
directions carefully.
Caution: Federal Law prohibits dispensing without prescription.
Store in a cool, dry place or at room teperature.
Notice to Dispenser: Patient
information Leaflet must be given
when dispensed to patient.
Advanced Pharmaceutical
Technology Inc.
Phone: (914) 358-5260
132 So.Central Ave., Elmsford, NY 10523
info@aptpharmatech.com
50mg Brand of
Estradiol Pellets
Lot No:
Exp. Date:
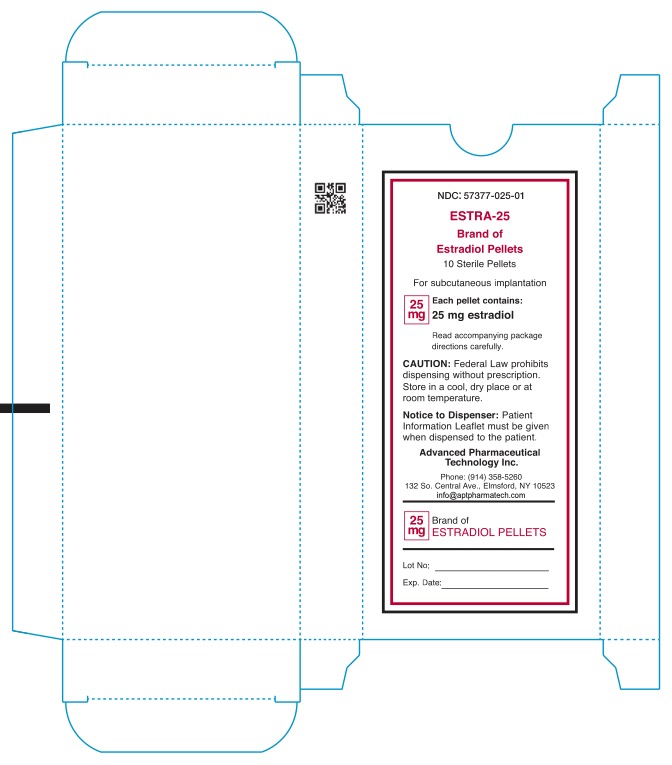
NDC: 57377-025-01
ESTRA-25
Brand of Estradiol Pellets
10 Sterile Pellets
For subcutaneous implantation
25mg
Each pellet contains:
25mg estradiol
Read accompanying package
directions carefully.
Caution: Federal Law prohibits dispensing without prescription.
Store in a cool, dry place or at room teperature.
Notice to Dispenser: Patient
information Leaflet must be given
when dispensed to patient.
Advanced Pharmaceutical
Technology Inc.
Phone: (914) 358-5260
132 So.Central Ave., Elmsford, NY 10523
infor@aptpharmatech.com
25mg Brand of
ESTRADIOL PELLETS
Lot No:
Exp date: