FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Epinephrine injection is indicated in the emergency treatment of allergic reactions (Type I) including anaphylaxis to stinging insects (e.g., order Hymenoptera, which includes bees, wasps, hornets, yellow jackets and fire ants), and biting insects (e.g., triatoma, mosquitoes), allergen immunotherapy, foods, drugs, diagnostic testing substances (e.g., radiocontrast media), and other allergens, as well as idiopathic anaphylaxis or exercise-induced anaphylaxis.
Epinephrine injection is intended for immediate administration in patients who are determined to be at increased risk for anaphylaxis, including individuals with a history of anaphylactic reactions.
Anaphylactic reactions may occur within minutes after exposure and consist of flushing, apprehension, syncope, tachycardia, thready or unobtainable pulse associated with a fall in blood pressure, convulsions, vomiting, diarrhea and abdominal cramps, involuntary voiding, wheezing, dyspnea due to laryngeal spasm, pruritus, rashes, urticaria, or angioedema.
Epinephrine injection is intended for immediate administration as emergency supportive therapy only and is not a replacement or substitute for immediate medical care.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage According to Patient Body Weight
- Patients greater than or equal to 30 kg (approximately 66 pounds or more): 0.3 mg
- Patients 15 kg to 30 kg (33 pounds to 66 pounds): 0.15 mg
2.2 Administration Instructions
- Inject the single-dose epinephrine injection intramuscularly or subcutaneously into the anterolateral aspect of the thigh, through clothing if necessary. Do not inject intravenously, and do not inject into buttocks, into digits, hands or feet [see Warnings and Precautions (5.2)].
- Instruct caregivers of young children who are prescribed an epinephrine injection and who may be uncooperative and kick or move during an injection to hold the leg firmly in place and limit movement prior to and during an injection [see Warnings and Precautions (5.2)].
- Each epinephrine injection is a single-dose of epinephrine injection for single use. Since the doses of epinephrine delivered from epinephrine injection are fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary.
- With severe persistent anaphylaxis, repeat injections with an additional epinephrine injection may be necessary. More than two sequential doses of epinephrine should only be administered under direct medical supervision [see Warnings and Precautions (5.1)].
- The epinephrine solution in the viewing window of epinephrine injection should be inspected visually for particulate matter and discoloration.
3 DOSAGE FORMS AND STRENGTHS
- Injection: 0.3 mg (0.3 mg/0.3 mL) of clear and colorless solution in single-dose pre-filled auto-injector
5 WARNINGS AND PRECAUTIONS
5.1 Emergency Treatment
Epinephrine injection is intended for immediate administration as emergency supportive therapy and is not intended as a substitute for immediate medical care. In conjunction with the administration of epinephrine, the patient should seek immediate medical or hospital care. More than two sequential doses of epinephrine should only be administered under direct medical supervision [see Indications and Usage (1), Dosage and Administration (2) and Patient Counseling Information (17)].
5.2 Injection-Related Complications
Epinephrine injection should only be injected into the anterolateral aspect of the thigh [see Dosage and Administration (2) and Patient Counseling Information (17)].
Do not inject intravenously
Large doses or accidental intravenous injection of epinephrine may result in cerebral hemorrhage due to a sharp rise in blood pressure. Rapidly acting vasodilators can counteract the marked pressor effects of epinephrine if there is such inadvertent administration.
Do not inject into buttock
Injection into the buttock may not provide effective treatment of anaphylaxis. Advise the patient to go immediately to the nearest emergency room for further treatment of anaphylaxis. Additionally, injection into the buttock has been associated with the development of Clostridial infections (gas gangrene). Cleansing with alcohol does not kill bacterial spores, and therefore, does not lower the risk.
Do not inject into digits, hands or feet
Since epinephrine is a strong vasoconstrictor, accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area. Advise the patient to go immediately to the nearest emergency room and to inform the healthcare provider in the emergency room of the location of the accidental injection. Treatment of such inadvertent administration should consist of vasodilation, in addition to further appropriate treatment of anaphylaxis [see Adverse Reactions (6)].
Hold leg firmly during injection
Lacerations, bent needles, and embedded needles have been reported when epinephrine has been injected into the thigh of young children who are uncooperative and kick or move during an injection. To minimize the risk of injection related injury when administering, hold the child’s leg firmly in place and limit movement prior to and during injection.
5.3 Serious Infections at the Injection Site
Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Clostridium spores can be present on the skin and introduced into the deep tissue with subcutaneous or intramuscular injection. While cleansing with alcohol may reduce presence of bacteria on the skin, alcohol cleansing does not kill Clostridium spores. To decrease the risk of Clostridium infection, do not inject epinephrine injection into the buttock [see Warnings and Precautions (5.2)]. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site.
5.4 Allergic Reactions Associated with Sulfite
The presence of a sulfite in this product should not deter administration of the drug for treatment of serious allergic or other emergency situations even if the patient is sulfite-sensitive.
Epinephrine is the preferred treatment for serious allergic reactions or other emergency situations even though this product contains sodium bisulfite, a sulfite that may, in other products, cause allergic-type reactions including anaphylactic symptoms or life-threatening or less severe asthmatic episodes in certain susceptible persons.
The alternatives to using epinephrine in a life-threatening situation may not be satisfactory.
5.5 Disease Interactions
Some patients may be at greater risk for developing adverse reactions after epinephrine administration. Despite these concerns, it should be recognized that the presence of these conditions is not a contraindication to epinephrine administration in an acute, life-threatening situation. Therefore, patients with these conditions, and/or any other person who might be in a position to administer epinephrine injection to a patient experiencing anaphylaxis should be carefully instructed in regard to the circumstances under which epinephrine should be used.
Patients with Heart Disease
Epinephrine should be administered with caution to patients who have heart disease, including patients with cardiac arrhythmias, coronary artery or organic heart disease, or hypertension. In such patients, or in patients who are on drugs that may sensitize the heart to arrhythmias, epinephrine may precipitate or aggravate angina pectoris as well as produce ventricular arrhythmias [see Drug Interactions (7) and Adverse Reactions (6)].
Other Patients and Diseases
Epinephrine should be administered with caution to patients with hyperthyroidism, diabetes, elderly individuals, and pregnant women. Patients with Parkinson’s disease may notice a temporary worsening of symptoms.
6 ADVERSE REACTIONS
Due to the lack of randomized, controlled clinical trials of epinephrine for the treatment of anaphylaxis, the true incidence of adverse reactions associated with the systemic use of epinephrine is difficult to determine. Adverse reactions reported in observational trials, case reports, and studies are listed below.
Common adverse reactions to systemically administered epinephrine include anxiety; apprehensiveness; restlessness; tremor; weakness; dizziness; sweating; palpitations; pallor; nausea and vomiting; headache; and/or respiratory difficulties. These symptoms occur in some persons receiving therapeutic doses of epinephrine, but are more likely to occur in patients with hypertension or hyperthyroidism [see Warnings and Precautions (5.5)].
Cardiovascular Reactions
- Arrhythmias, including fatal ventricular fibrillation, have been reported, particularly in patients with underlying cardiac disease or those receiving certain drugs [see Warnings and Precautions (5.5) and Drug Interactions (7)].
- Rapid rises in blood pressure have produced cerebral hemorrhage, particularly in elderly patients with cardiovascular disease [see Warnings and Precautions (5.5)].
- Angina may occur in patients with coronary artery disease [see Warnings and Precautions (5.5)].
- Rare cases of stress cardiomyopathy have been reported in patients treated with epinephrine.
Reactions from Accidental Injection and/or Improper Technique
- Accidental injection into the digits, hands or feet may result in loss of blood flow to the affected area [see Warnings and Precautions (5.2)].
- Adverse reactions experienced as a result of accidental injections may include increased heart rate, local reactions including injection site pallor, coldness and hypoesthesia or injury at the injection site resulting in bruising, bleeding, discoloration, erythema or skeletal injury.
- Lacerations, bent needles, and embedded needles have been reported when epinephrine injection has been injected into the thigh of young children who are uncooperative and kick or move during an injection [see Warnings and Precautions (5.2)].
- Injection into the buttock has resulted in cases of gas gangrene [see Warnings and Precautions (5.2)].
Skin and Soft Tissue Infections
- Rare cases of serious skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported following epinephrine injection in the thigh [see Warnings and Precautions (5.3)].
7 DRUG INTERACTIONS
Cardiac Glycosides, Diuretics, and Anti-arrhythmics
Patients who receive epinephrine while concomitantly taking cardiac glycosides, diuretics, or anti-arrhythmics should be observed carefully for the development of cardiac arrhythmias [see Warnings and Precautions (5.5)].
Antidepressants, Monoamine Oxidase Inhibitors, Levothyroxine, and Antihistamines
The effects of epinephrine may be potentiated by tricyclic antidepressants, monoamine oxidase inhibitors, levothyroxine sodium, and certain antihistamines, notably chlorpheniramine, tripelennamine, and diphenhydramine.
Beta-Adrenergic Blockers
The cardiostimulating and bronchodilating effects of epinephrine are antagonized by beta- adrenergic blocking drugs, such as propranolol.
Alpha-Adrenergic Blockers
The vasoconstricting and hypertensive effects of epinephrine are antagonized by alpha-adrenergic blocking drugs, such as phentolamine.
Ergot Alkaloids
Ergot alkaloids may also reverse the pressor effects of epinephrine.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available human data on the use of epinephrine injection in pregnant women to inform a drug-associated risk of adverse developmental outcomes. In animal reproduction studies, epinephrine administered by the subcutaneous route to rabbits, mice, and hamsters during the period of organogenesis was teratogenic at doses 7 times and higher than the maximum recommended daily subcutaneous or intramuscular dose on a mg/m2 basis (see Data). Epinephrine is the first-line medication of choice for the treatment of anaphylaxis during pregnancy in humans. Epinephrine should be used for treatment of anaphylaxis during pregnancy in the same manner as it is used in non-pregnant patients.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk:
During pregnancy, anaphylaxis can be catastrophic and can lead to hypoxic-ischemic encephalopathy and permanent central nervous system damage or death in the mother and, more commonly, in the fetus or neonate. The prevalence of anaphylaxis occurring during pregnancy is reported to be approximately 3 cases per 100,000 deliveries.
Management of anaphylaxis during pregnancy is similar to management in the general population. Epinephrine is the first line-medication of choice for treatment of anaphylaxis; it should be used in the same manner in pregnant and non-pregnant patients. In conjunction with the administration of epinephrine, the patient should seek immediate medical or hospital care.
Data
Animal Data
In an embryofetal development study with rabbits dosed during the period of organogenesis, epinephrine was shown to be teratogenic (including gastroschisis and embryonic lethality) at doses approximately 40 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 1.2 mg/kg/day for two to three days).
In an embryofetal development study with mice dosed during the period of organogenesis, epinephrine was shown to be teratogenic (including embryonic lethality) at doses approximately 8 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at maternal subcutaneous dose of 1 mg/kg/day for 10 days). These effects were not seen in mice at approximately 4 times the maximum recommended daily intramuscular or subcutaneous dose (on a mg/m2 basis at a subcutaneous maternal dose of 0.5 mg/kg/day for 10 days).
In an embryofetal development study with hamsters dosed during the period of organogenesis from gestation days 7 to 10, epinephrine was shown to be teratogenic at doses approximately 7 times the maximum recommended intramuscular or subcutaneous dose (on a mg/m2 basis at a maternal subcutaneous dose of 0.5 mg/kg/day).
8.2 Lactation
Risk Summary
There are no data on the presence of epinephrine in human milk, or the effects of epinephrine on the breastfed infant or on milk production. Epinephrine is the first line-medication of choice for treatment of anaphylaxis; it should be used in the same manner in breastfeeding and no-breastfeeding patients.
8.4 Pediatric Use
Epinephrine injection may be administered to pediatric patients at a dosage appropriate to body weight [see Dosage and Administration (2.1)]. Clinical experience with the use of epinephrine suggests that the adverse reactions seen in children are similar in nature and extent to those both expected and reported in adults. Since the dose of epinephrine delivered from epinephrine injection is fixed, consider using other forms of injectable epinephrine if doses lower than 0.15 mg are deemed necessary.
8.5 Geriatric Use
Clinical studies for the treatment of anaphylaxis have not been performed in subjects aged 65 and over to determine whether they respond differently from younger subjects. However, other reported clinical experience with use of epinephrine for the treatment of anaphylaxis has identified that geriatric patients may be particularly sensitive to the effects of epinephrine. Therefore, epinephrine injection should be administered with caution in elderly individuals, who may be at greater risk for developing adverse reactions after epinephrine administration [see Warnings and Precautions (5.5) and Overdosage (10)].
10 OVERDOSAGE
Overdosage of epinephrine may produce extremely elevated arterial pressure, which may result in cerebrovascular hemorrhage, particularly in elderly patients. Overdosage may also result in pulmonary edema because of peripheral vascular constriction together with cardiac stimulation. Treatment consists of a rapidly acting vasodilators or alpha-adrenergic blocking drugs and/or respiratory support.
Epinephrine overdosage can also cause transient bradycardia followed by tachycardia, and these may be accompanied by potentially fatal cardiac arrhythmias. Premature ventricular contractions may appear within one minute after injection and may be followed by multifocal ventricular tachycardia (prefibrillation rhythm). Subsidence of the ventricular effects may be followed by atrial tachycardia and occasionally by atrioventricular block. Treatment of arrhythmias consists of administration of a beta-adrenergic blocking drug such as propranolol.
Overdosage sometimes results in extreme pallor and coldness of the skin, metabolic acidosis, and kidney failure. Suitable corrective measures must be taken in such situations.
11 DESCRIPTION
Epinephrine injection, USP 0.3 mg is an auto-injector and a combination product containing drug and device components.
Each epinephrine injection, USP 0.3 mg delivers a single dose of 0.3 mg epinephrine from epinephrine injection, USP (0.3 mL) in a sterile solution.
Epinephrine injection, USP 0.3 mg contain 1.1 mL of epinephrine solution. 0.3 mL epinephrine solution is dispensed for epinephrine injection, USP 0.3 mg when activated. The solution remaining after activation is not available for future use and should be discarded.
Each 0.3 mL in epinephrine injection, USP 0.3 mg contains 0.3 mg epinephrine, 2.6 mg sodium chloride, not more than 1.5 mg chlorobutanol, 0.45 mg sodium bisulfite, hydrochloric acid and sodium hydroxide to adjust pH, and water for injection. The pH range is 2.2-5.0.
Epinephrine is a sympathomimetic catecholamine. Chemically, epinephrine is (-)-3,4-Dihydroxy-α-[(methylamino)methyl]benzyl alcohol with the following structure:
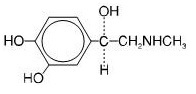
Epinephrine solution deteriorates rapidly on exposure to air or light, turning pink from oxidation to adrenochrome and brown from the formation of melanin. Replace epinephrine injection, USP if the epinephrine solution appears discolored (pinkish or brown color), cloudy, or contains particles.
Thoroughly review the patient instructions and operation of epinephrine injection, USP with patients and caregivers prior to use [see Patient Counseling Information (17)].
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Through its action on alpha-adrenergic receptors, epinephrine lessens the vasodilation and increased vascular permeability that occurs during anaphylaxis, which can lead to loss of intravascular fluid volume and hypotension.
Through its action on beta-adrenergic receptors, epinephrine causes bronchial smooth muscle relaxation and helps alleviate bronchospasm, wheezing, and dyspnea that may occur during anaphylaxis.
Epinephrine also alleviates pruritus, urticaria, and angioedema, and may relieve gastrointestinal and genitourinary symptoms associated with anaphylaxis because of its relaxer effects on the smooth muscle of the stomach, intestine, uterus and urinary bladder.
When given intramuscularly or subcutaneously, epinephrine has a rapid onset and short duration of action.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to evaluate the carcinogenic potential of epinephrine have not been conducted.
Epinephrine and other catecholamines have been shown to have mutagenic potential in vitro. Epinephrine was positive in the Salmonella bacterial reverse mutation assay, positive in the mouse lymphoma assay, and negative in the in vivo micronucleus assay. Epinephrine is an oxidative mutagen based on the E. coli WP2 Mutoxitest bacterial reverse mutation assay. This should not prevent the use of epinephrine where indicated under the conditions noted under [see Indications and Usage (1)].
The potential for epinephrine to impair reproductive performance has not been evaluated, but epinephrine has been shown to decrease implantation in female rabbits dosed subcutaneously with 1.2 mg/kg/day (40-fold the highest human intramuscular or subcutaneous daily dose) during gestation days 3 to 9.
16 HOW SUPPLIED/STORAGE AND HANDLING
Storage and Handling
Protect from light. Epinephrine is light sensitive and should be stored in the carrying-case provided to protect it from light. Store at room temperature (20°C to 25°C (68°F to 77°F)); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature]. Do not refrigerate. Before using, check to make sure the solution in the auto-injector is clear and colorless. Replace the auto-injector if the solution is discolored (pinkish or brown color), cloudy, or contains particles.
Properly dispose of all used, unwanted, or expired epinephrine injection, USP.
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information and Instructions for Use)
A healthcare provider should review the patient instructions and operation of epinephrine injection, in detail, with the patient or caregiver.
Epinephrine is essential for the treatment of anaphylaxis. Carefully instruct patients who are at risk of or with a history of severe allergic reactions (anaphylaxis) to insect stings or bites, foods, drugs, and other allergens, as well as idiopathic and exercise-induced anaphylaxis, about the circumstances under which epinephrine should be used.
Administration
Instruct patients and/or caregivers in the appropriate use of epinephrine injection. Epinephrine injection should be injected into the middle of the outer thigh (through clothing if necessary).
Instruct caregivers to hold the leg of young children firmly in place and limit movement prior to and during injection. Lacerations, bent needles, and embedded needles have been reported when epinephrine injection has been injected into the thigh of young children who are uncooperative and kick during an injection [see Warnings and Precautions (5.2)].
Advise patients to seek immediate medical care in conjunction with administration of epinephrine injection.
Complete patient information, including dosage, directions for proper administration and precautions can be found inside each epinephrine injection carton. A printed label on the surface of epinephrine injection shows instructions for use and a diagram depicting the injection process.
Training
Instruct patients and/or caregivers to use the Trainer to familiarize themselves with the use of epinephrine injection in an allergic emergency. The Trainer may be used multiple times.
Adverse Reactions
Epinephrine may produce symptoms and signs that include an increase in heart rate, the sensation of a more forceful heartbeat, palpitations, sweating, nausea and vomiting, difficulty breathing, pallor, dizziness, weakness or shakiness, headache, apprehension, nervousness, or anxiety. These signs and symptoms usually subside rapidly, especially with rest, quiet, and recumbency. Patients with hypertension or hyperthyroidism may develop more severe or persistent effects, and patients with coronary artery disease could experience angina. Patients with diabetes may develop increased blood glucose levels following epinephrine administration. Patients with Parkinson’s disease may notice a temporary worsening of symptoms [see Warnings and Precautions (5.5)].
Accidental Injection
Advise patients to seek immediate medical care in the case of accidental injection. Since epinephrine is a strong vasoconstrictor when injected into the digits, hands or feet, treatment should be directed at vasodilation if there is such an accidental injection to these areas [see Warnings and Precautions (5.2)].
Serious Infections at the Injection Site
Rare cases of skin and soft tissue infections, including necrotizing fasciitis and myonecrosis caused by Clostridia (gas gangrene), have been reported at the injection site following epinephrine injection for anaphylaxis. Advise patients to seek medical care if they develop signs or symptoms of infection, such as persistent redness, warmth, swelling, or tenderness, at the epinephrine injection site [see Warnings and Precautions (5.3)].
Pregnancy and Breastfeeding
Inform patients that epinephrine injection has not been studied in pregnant women or breastfeeding mothers so the effects of epinephrine injection on pregnant women or breastfed infants are not known. Instruct patients to tell their healthcare provider if they are pregnant, become pregnant, or are thinking about becoming pregnant. Instruct patients to tell their healthcare provider if they plan to breastfeed their infant [see Use in Specific Populations (8.1, 8.2)].
Storage and Handling
Instruct patients to inspect the epinephrine solution visually through the viewing window periodically. Replace epinephrine injection, USP auto-injector if the epinephrine solution appears discolored (pinkish or brown), cloudy, or contains particles. Epinephrine is light sensitive, store in the outer case provided to protect it from light. Instruct patients that epinephrine injection, USP auto-injector must be properly disposed of once the blue caps have been removed or after use [see How Supplied/Storage and Handling (16)].
Complete patient information, including dosage, directions for proper administration and precautions are provided inside each epinephrine injection carton.
Manufactured by:
Hospira, Inc.
McPherson, KS 67460
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
© 2021 Amneal Pharmaceuticals LLC. All rights reserved.
For inquiries call 1-877-835-5472
Patient Information
EPINEPHRINE injection (ep-in-eph-rine),
for intramuscular or subcutaneous use
For allergic emergencies (anaphylaxis)
Read this Patient Information leaflet carefully before you use epinephrine injection, and each time you get a refill. There may be new information. You, your parent, caregiver, or others who may be in a position to administer epinephrine injection should know how to use it before you have an allergic emergency.
This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about epinephrine injection?
1. Epinephrine injection contains epinephrine, a medicine used to treat allergic emergencies (anaphylaxis). Anaphylaxis can be life-threatening, can happen within minutes, and can be caused by stinging and biting insects, allergy injections, foods, medicines, exercise, or other unknown causes. Symptoms of an anaphylaxis may include:
- trouble breathing
- wheezing
- hoarseness (changes in the way your voice sounds)
- hives (raised reddened rash that may itch)
- severe itching
- swelling of your face, lips, mouth, or tongue
- skin rash, redness, or swelling
- fast heartbeat
- weak pulse
- feeling very anxious
- confusion
- stomach pain
- losing control of urine or bowel movements (incontinence)
- diarrhea or stomach cramps
- dizziness, fainting, or “passing out” (unconsciousness)
2. Always carry your epinephrine injection with you because you may not know when anaphylaxis may happen. Talk to your healthcare provider if you need additional units to keep at work, school, or other locations. Tell your family members, caregivers, and others where you keep your epinephrine injection and how to use it before you need it. You may be unable to speak in an allergic emergency.
3. When you have an allergic emergency (anaphylaxis)
- Use epinephrine injection right away.
- Get emergency medical help right away. You may need further medical attention. You may need to use a second epinephrine injection if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode.
What is epinephrine injection?
- Epinephrine injection is a disposable, prefilled automatic injection device (auto-injector) used to treat life-threatening, allergic emergencies including anaphylaxis in people who are at risk for or have a history of serious allergic emergencies. Each device contains a single dose of epinephrine.
- Epinephrine injection is for immediate self (or caregiver) administration and does not take the place of emergency medical care. You should get emergency medical help right away after using epinephrine injection.
- Epinephrine injection is for people who have been prescribed this medicine by their healthcare provider.
- The epinephrine injection 0.3 mg auto-injector is for patients who weigh 66 pounds or more (30 kilograms or more).
- It is not known if epinephrine injection is safe and effective in children who weigh less than 33 pounds (15 kilograms).
Before using epinephrine injection, tell your healthcare provider about all your medical conditions, especially if you:
- have heart problems or high blood pressure
- have diabetes
- have thyroid problems
- have asthma
- have a history of depression
- have Parkinson's disease
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if epinephrine will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if epinephrine passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Tell your healthcare provider of all known allergies.
Especially tell your healthcare provider if you take certain asthma medicines.
Epinephrine injection and other medicines may affect each other, causing side effects. Epinephrine injection may affect the way other medicines work, and other medicines may affect how epinephrine injection works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
Use your epinephrine injection for treatment of anaphylaxis as prescribed by your healthcare provider, regardless of your medical conditions or the medicine you take.
How should I use epinephrine injection?
- Each epinephrine injection contains only 1 dose of medicine.
- Epinephrine injection should only be injected into the middle of the outer thigh (upper leg). It can be injected through clothing, if needed.
- Read the Instructions for Use at the end of this Patient Information Leaflet for information about the right way to use epinephrine injection.
- Your healthcare provider will show you how to safely use epinephrine injection.
- Use epinephrine injection exactly as your healthcare provider tells you to use it. You may need to use a second epinephrine injection if symptoms continue or recur. Only a healthcare provider should give additional doses of epinephrine if you need more than 2 injections for a single anaphylaxis episode.
- Caution: Never put your thumb, fingers, or hand over the red tip. Never press or push the red tip with your thumb, fingers, or hand. The needle comes out of the red tip. Accidental injection into finger, hands, or feet may cause a loss of blood flow to those areas. If this happens, go immediately to the nearest emergency room. Tell the healthcare provider where on your body you received the accidental injection.
- Your epinephrine injection comes packaged in a carton containing 1 or 2 epinephrine injection.
- You may request a separate Trainer, that comes packaged with instructions. Additional video instructions on the use of epinephrine injection are available from www.epinephrineautoinject.com. The epinephrine injection, USP auto-injector Trainer has a beige color. The beige epinephrine injection Trainer contains no medicine and no needle. Practice with your epinephrine injection Trainer before an allergic emergency happens to make sure you are able to safely use the real epinephrine injection in an emergency. Always carry your real epinephrine injection with you in case of an allergic emergency.
- Do not drop the carrying case or epinephrine injection. If the carrying case or epinephrine injection is dropped, check for damage and leakage. Throw away (dispose of) epinephrine injection and the carrying case, and replace if damage or leakage is noticed or suspected.
What are the possible side effects of epinephrine injection?
Epinephrine injection may cause serious side effects.
-
Epinephrine injection should only be injected into the middle of your outer thigh (upper leg). Do not inject epinephrine injection into your:
- veins
- buttocks
- fingers, toes, hands or feet.
If you accidentally inject epinephrine injection into any other part of your body, go to the nearest emergency room right away. Tell the healthcare provider where on your body you received the accidental injection.
-
Rarely, people who use epinephrine injection may develop infections at the injection site within a few days of an injection. Some of these infections can be serious. Call your healthcare provider right away if you have any of the following at an injection site:
- redness that does not go away
- swelling
- tenderness
- the area feels warm to the touch
- Cuts on the skin, bent needles, and needles that remain in the skin after the injection, have happened in young children who do not cooperate and kick or move during an injection. If you inject a young child with epinephrine injection, hold their leg firmly in place before and during the injection to prevent injuries. Ask your healthcare provider to show you how to properly hold the leg of a young child during an injection.
- If you have certain medical conditions, or take certain medicines, your condition may get worse or you may have more or longer lasting side effects when you use epinephrine injection. Talk to your healthcare provider about all your medical conditions.
Common side effects of epinephrine injection include:
- faster, irregular or “pounding” heartbeat
- sweating
- headache
- weakness
- shakiness
- paleness
- feelings of over excitement, nervousness, or anxiety
- dizziness
- nausea or vomiting
- breathing problems
These side effects may go away with rest. Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of epinephrine injection. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store epinephrine injection?
- Store epinephrine injection at room temperature between 68°F to 77° F (20°C to 25° C).
- Protect from light.
- Do not expose to extreme heat or cold. For example, do not store in your vehicle’s glove box and do not store in the refrigerator or freezer.
- Examine the contents in the clear viewing window of your epinephrine injection periodically. The solution should be clear. If the solution is discolored (pinkish or brown), cloudy or contains solid particles, replace the unit.
- Always keep your epinephrine injection in the carrying case to protect it from damage. The carrying case is not waterproof.
- The two blue end caps help to prevent accidental injection. Do not remove the blue end caps until you are ready to use epinephrine injection.
- Your epinephrine injection has an expiration date. Replace it before the expiration date.
- Throw away (dispose of) expired, unwanted, or unused epinephrine injections in an FDA-cleared sharps disposal container. Do not throw away epinephrine injection in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- Made of heavy-duty plastic,
- Can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- Upright and stable during use,
- Leak-resistant, and
- Properly labeled to warn of hazardous waste inside the container.
Keep epinephrine injection and all medicines out of the reach of children.
General information about the safe and effective use of epinephrine injection:
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use epinephrine injection for a condition for which it was not prescribed. Do not give epinephrine injection to other people.
This Patient Information Leaflet summarizes the most important information about epinephrine injection. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about epinephrine injection that is written for health professionals.
What are the ingredients in epinephrine injection?
Active Ingredient: epinephrine
Inactive Ingredients: sodium chloride, chlorobutanol, sodium bisulfite, hydrochloric acid and sodium hydroxide, and water.
For more information and video instructions on the use of epinephrine injection go to www.epinephrineautoinject.com or call 1-877-835-5472.
Important Information
- The epinephrine injection 0.3 mg has a yellow colored label.
- The epinephrine injection Trainer has a beige color, and contains no medicine and no needle.
- Your epinephrine injection is designed to work through clothing.
- The two blue end caps on epinephrine injection help to prevent accidental injection of the device. Do not remove the blue end caps until you are ready to use it.
- Only inject into the middle of the outer thigh (upper leg). Never inject into any other part of the body.
- Never put your thumb, fingers, or your hand over the red tip. The needle comes out of the red tip.
- If an accidental injection happens, get medical help right away.
- Do not place patient information or any other foreign objects in carrier with the epinephrine injection, as this may prevent you from removing the auto-injector for use.
This Patient Information has been approved by the U.S. Food and Drug Administration
Rev. 02-2021-03
Instructions for Use
EPINEPHRINE injection (ep-in-eph-rine)
for intramuscular or subcutaneous use
For allergic emergencies (anaphylaxis)
Read this Instructions for Use carefully before you use epinephrine injection and each time you get a refill. There may be new information. Before you need to use your epinephrine injection, make sure your healthcare provider shows you the right way to use it. Parents, caregivers, and others who may be in a position to administer epinephrine injection should also understand how to use it well. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. If you have any questions, ask your healthcare provider.
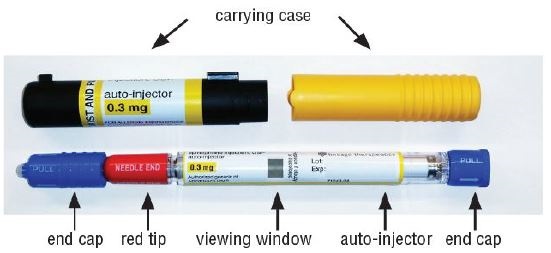
Your epinephrine injection
Step 1. Prepare epinephrine injection for injection
- Remove epinephrine injection from its protective carrying case.
- Pull off blue end caps. You will now see a red tip. Grasp the epinephrine injection in your fist with the red tip pointing downward. See Figure A.
Note:
- The needle comes out of the red tip.
- To avoid an accidental injection, never put your thumb, fingers, or hand over the red tip. If an accidental injection happens, get medical help right away.

Step 2. Administer epinephrine injection
- If you are administering epinephrine injection to a young child, hold the leg firmly in place and limit movement prior to and while administering an injection.
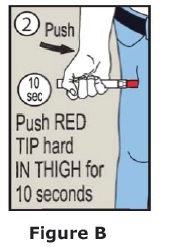
- Put the red tip against the middle of the outer thigh (upper leg) at a 90° angle (perpendicular) to the thigh.
- Press down hard and hold firmly against the thigh for approximately 10 seconds to deliver the medicine. See Figure B.
- Only inject into the middle of the outer thigh. Do not inject into any other part of the body.
- Remove epinephrine injection from the thigh.
- Massage the area for 10 seconds.
- Check the red tip. The injection is complete and you have received the correct dose of the medicine if you see the needle sticking out of the red tip. If you do not see the needle repeat Step 2.
Step 3. Get emergency medical help right away. You may need further medical attention. You may need to use a second epinephrine injection if symptoms continue or recur.
Step 4. After use Disposal
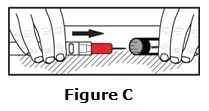
Carefully cover the needle with the carrying case.
- Lay the labeled half of the carrying case cover down on a flat surface. Use one hand to carefully slide the end of the epinephrine injection needle first, into the labeled carrying case cover. See Figure C.
- After the needle is inside the labeled cover, push the unlabeled half of the carrying case cover firmly over the non-needle end of the epinephrine injection . See Figure D.
- Take your used epinephrine injection with you when you go to see a healthcare provider.
- Tell the healthcare provider that you have received an injection of epinephrine. Show the healthcare provider where you received the injection.
- Give your used epinephrine injection to the healthcare provider for inspection and proper disposal.
- Ask for a refill, if needed.
Note:
- Epinephrine injection is a single-use injectable device that delivers a fixed dose of epinephrine. Epinephrine injection cannot be reused. Do not attempt to reuse epinephrine injection after the device has been activated. It is normal for most of the medicine to remain in the auto-injector after the dose is injected. The correct dose has been administered if you see the needle sticking out of the red tip.
- A separate epinephrine injection Trainer is available. The epinephrine injection Trainer has a beige color. The beige epinephrine injection Trainer contains no medicine and no needle. Practice with your epinephrine injection Trainer, but always carry your real epinephrine injection in case of an allergic emergency.
- If you will be administering epinephrine injection to a young child, ask your healthcare provider to show you how to properly hold the leg in place while administering a dose.
- Do not try to take epinephrine injection.
For more information and video instructions on the use of epinephrine injection, go to www.epinephrineautoinject.com or call 1-877-835-5472.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
© 2021 Amneal Pharmaceuticals LLC. All rights reserved.
Manufactured by:
Hospira, Inc.
McPherson, KS 67460
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Revised 02-2021-03
Albuterol Sulfate Inhalation Aerosol HFA with Dose Indicator
FOR ORAL INHALATION ONLY
Prescribing Information
DESCRIPTION
The active component of Albuterol Sulfate Inhalation Aerosol is albuterol sulfate, USP racemic α 1 [( tert-Butylamino)methyl]-4-hydroxy- m-xylene-α,α'-diol sulfate (2:1)(salt), a relatively selective beta 2-adrenergic bronchodilator having the following chemical structure:
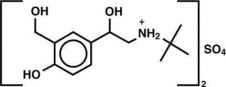
Albuterol sulfate is the official generic name in the United States. The World Health Organization recommended name for the drug is salbutamol sulfate. The molecular weight of albuterol sulfate is 576.7, and the empirical formula is (C13H21NO3)2•H2SO4. Albuterol sulfate is a white to off-white crystalline solid. It is soluble in water and slightly soluble in ethanol. Albuterol Sulfate Inhalation Aerosol is a pressurized metered-dose aerosol unit for oral inhalation. It contains a microcrystalline suspension of albuterol sulfate in propellant HFA-134a (1,1,1,2-tetrafluoroethane), ethanol, and oleic acid.
Each actuation delivers 120 mcg albuterol sulfate, USP from the valve and 108 mcg albuterol sulfate, USP from the mouthpiece (equivalent to 90 mcg of albuterol base from the mouthpiece). Each canister provides 200 inhalations. It is recommended to prime the inhaler before using for the first time and in cases where the inhaler has not been used for more than 2 weeks by releasing four “test sprays” into the air, away from the face.
This product does not contain chlorofluorocarbons (CFCs) as the propellant.
CLINICAL PHARMACOLOGY
Mechanism of Action In vitro studies and in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on beta 2-adrenergic receptors compared with isoproterenol. While it is recognized that beta 2-adrenergic receptors are the predominant receptors on bronchial smooth muscle, data indicate that there is a population of beta 2-receptors in the human heart existing in a concentration between 10% and 50% of cardiac beta-adrenergic receptors. The precise function of these receptors has not been established. (See WARNINGS, Cardiovascular Effects section.)
Activation of beta 2-adrenergic receptors on airway smooth muscle leads to the activation of adenylcyclase and to an increase in the intracellular concentration of cyclic-3',5'-adenosine monophosphate (cyclic AMP). This increase of cyclic AMP leads to the activation of protein kinase A, which inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in relaxation. Albuterol relaxes the smooth muscles of all airways, from the trachea to the terminal bronchioles. Albuterol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. Increased cyclic AMP concentrations are also associated with the inhibition of release of mediators from mast cells in the airway.
Albuterol has been shown in most clinical trials to have more effect on the respiratory tract, in the form of bronchial smooth muscle relaxation, than isoproterenol at comparable doses while producing fewer cardiovascular effects. Controlled clinical studies and other clinical experience have shown that inhaled albuterol, like other beta-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes.
Preclinical Intravenous studies in rats with albuterol sulfate have demonstrated that albuterol crosses the blood-brain barrier and reaches brain concentrations amounting to approximately 5% of the plasma concentrations. In structures outside the blood-brain barrier (pineal and pituitary glands), albuterol concentrations were found to be 100 times those in the whole brain.
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta 2-agonist and methylxanthines were administered concurrently. The clinical significance of these findings is unknown.
Propellant HFA-134a is devoid of pharmacological activity except at very high doses in animals (380-1300 times the maximum human exposure based on comparisons of AUC values), primarily producing ataxia, tremors, dyspnea, or salivation. These are similar to effects produced by the structurally related chlorofluorocarbons (CFCs), which have been used extensively in metered dose inhalers.
In animals and humans, propellant HFA-134a was found to be rapidly absorbed and rapidly eliminated, with an elimination half-life of 3 to 27 minutes in animals and 5 to 7 minutes in humans. Time to maximum plasma concentration (Tmax) and mean residence time are both extremely short, leading to a transient appearance of HFA-134a in the blood with no evidence of accumulation.
Pharmacokinetics In a single-dose bioavailability study which enrolled six healthy, male volunteers, transient low albuterol levels (close to the lower limit of quantitation) were observed after administration of two puffs from both Albuterol Sulfate Inhalation Aerosol and a CFC 11/12 propelled albuterol inhaler. No formal pharmacokinetic analyses were possible for either treatment, but systemic albuterol levels appeared similar.
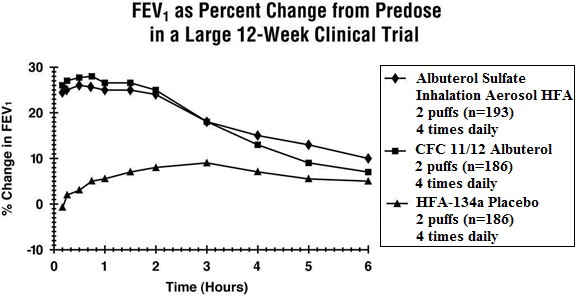
Clinical Trials In a 12-week, randomized, double-blind, double-dummy, active- and placebo-controlled trial, 565 patients with asthma were evaluated for the bronchodilator efficacy of Albuterol Sulfate Inhalation Aerosol (193 patients) in comparison to a CFC 11/12 propelled albuterol inhaler (186 patients) and an HFA-134a placebo inhaler (186 patients).
Serial FEV 1 measurements (shown below as percent change from test-day baseline) demonstrated that two inhalations of Albuterol Sulfate Inhalation Aerosol produced significantly greater improvement in pulmonary function than placebo and produced outcomes which were clinically comparable to a CFC 11/12 propelled albuterol inhaler.
The mean time to onset of a 15% increase in FEV 1 was 6 minutes and the mean time to peak effect was 50 to 55 minutes. The mean duration of effect as measured by a 15% increase in FEV 1 was 3 hours. In some patients, duration of effect was as long as 6 hours.
In another clinical study in adults, two inhalations of Albuterol Sulfate Inhalation Aerosol taken 30 minutes before exercise prevented exercise-induced bronchospasm as demonstrated by the maintenance of FEV 1 within 80% of baseline values in the majority of patients.
In a 4-week, randomized, open-label trial, 63 children, 4 to 11 years of age, with asthma were evaluated for the bronchodilator efficacy of Albuterol Sulfate Inhalation Aerosol (33 pediatric patients) in comparison to a CFC 11/12 propelled albuterol inhaler (30 pediatric patients).
Serial FEV 1 measurements as percent change from test-day baseline demonstrated that two inhalations of Albuterol Sulfate Inhalation Aerosol produced outcomes which were clinically comparable to a CFC 11/12 propelled albuterol inhaler.
The mean time to onset of a 12% increase in FEV 1 for Albuterol Sulfate Inhalation Aerosol was 7 minutes and the mean time to peak effect was approximately 50 minutes. The mean duration of effect as measured by a 12% increase in FEV 1 was 2.3 hours. In some pediatric patients, duration of effect was as long as 6 hours.
In another clinical study in pediatric patients, two inhalations of Albuterol Sulfate Inhalation Aerosol taken 30 minutes before exercise provided comparable protection against exercise-induced bronchospasm as a CFC 11/12 propelled albuterol inhaler.
INDICATIONS AND USAGE
Albuterol Sulfate Inhalation Aerosol is indicated in adults and children 4 years of age and older for the treatment or prevention of bronchospasm with reversible obstructive airway disease and for the prevention of exercise-induced bronchospasm.
CONTRAINDICATIONS
Albuterol Sulfate Inhalation Aerosol is contraindicated in patients with a history of hypersensitivity to albuterol or any other Albuterol Sulfate Inhalation Aerosol components.
WARNINGS
- Paradoxical Bronchospasm: Inhaled albuterol sulfate can produce paradoxical bronchospasm that may be life threatening. If paradoxical bronchospasm occurs, Albuterol Sulfate Inhalation Aerosol should be discontinued immediately and alternative therapy instituted. It should be recognized that paradoxical bronchospasm, when associated with inhaled formulations, frequently occurs with the first use of a new canister.
- Deterioration of Asthma: Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of Albuterol Sulfate Inhalation Aerosol than usual, this may be a marker of destabilization of asthma and requires re-evaluation of the patient and treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
- Use of Anti-inflammatory Agents: The use of beta-adrenergic-agonist bronchodilators alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, e.g., corticosteroids, to the therapeutic regimen.
-
Cardiovascular Effects: Albuterol Sulfate Inhalation Aerosol, like other beta-adrenergic agonists, can produce clinically significant cardiovascular effects in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of Albuterol Sulfate Inhalation Aerosol at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce ECG changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, Albuterol Sulfate Inhalation Aerosol, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
- Do Not Exceed Recommended Dose: Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs in patients with asthma. The exact cause of death is unknown, but cardiac arrest following an unexpected development of a severe acute asthmatic crisis and subsequent hypoxia is suspected.
- Immediate Hypersensitivity Reactions: Immediate hypersensitivity reactions may occur after administration of albuterol sulfate, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema.
PRECAUTIONS
General Albuterol sulfate, as with all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension; in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus; and in patients who are unusually responsive to sympathomimetic amines. Clinically significant changes in systolic and diastolic blood pressure have been seen in individual patients and could be expected to occur in some patients after use of any beta-adrenergic bronchodilator.
Large doses of intravenous albuterol have been reported to aggravate preexisting diabetes mellitus and ketoacidosis. As with other beta-agonists, albuterol may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease is usually transient, not requiring supplementation.
Information for Patients See illustrated Patient's Instructions for Use. SHAKE WELL BEFORE USING. Patients should be given the following information:
It is recommended to prime the inhaler before using for the first time and in cases where the inhaler has not been used for more than 2 weeks by releasing four “test sprays” into the air, away from the face.
KEEPING THE PLASTIC MOUTHPIECE CLEAN IS VERY IMPORTANT TO PREVENT MEDICATION BUILDUP AND BLOCKAGE. THE MOUTHPIECE SHOULD BE WASHED, SHAKEN TO REMOVE EXCESS WATER, AND AIR DRIED THOROUGHLY AT LEAST ONCE A WEEK. INHALER MAY CEASE TO DELIVER MEDICATION IF NOT PROPERLY CLEANED.
The mouthpiece should be cleaned (with the canister removed) by running warm water through the top and bottom for 30 seconds at least once a week. The mouthpiece must be shaken to remove excess water, then air dried thoroughly (such as overnight). Blockage from medication buildup or improper medication delivery may result from failure to thoroughly air dry the mouthpiece.
If the mouthpiece should become blocked (little or no medication coming out of the mouthpiece), the blockage may be removed by washing as described above.
If it is necessary to use the inhaler before it is completely dry, shake off excess water, replace canister, test spray twice away from face, and take the prescribed dose. After such use, the mouthpiece should be rewashed and allowed to air dry thoroughly.
The action of Albuterol Sulfate Inhalation Aerosol should last up to 4 to 6 hours. Albuterol Sulfate Inhalation Aerosol should not be used more frequently than recommended. Do not increase the dose or frequency of doses of Albuterol Sulfate Inhalation Aerosol without consulting your physician. If you find that treatment with Albuterol Sulfate Inhalation Aerosol becomes less effective for symptomatic relief, your symptoms become worse, and/or you need to use the product more frequently than usual, medical attention should be sought immediately. While you are taking Albuterol Sulfate Inhalation Aerosol, other inhaled drugs and asthma medications should be taken only as directed by your physician.
Common adverse effects of treatment with inhaled albuterol include palpitations, chest pain, rapid heart rate, tremor, or nervousness. If you are pregnant or nursing, contact your physician about use of Albuterol Sulfate Inhalation Aerosol. Effective and safe use of Albuterol Sulfate Inhalation Aerosol includes an understanding of the way that it should be administered. Use Albuterol Sulfate Inhalation Aerosol only with the actuator supplied with the product. Discard the canister after 200 sprays have been used.
In general, the technique for administering Albuterol Sulfate Inhalation Aerosol to children is similar to that for adults. Children should use Albuterol Sulfate Inhalation Aerosol under adult supervision, as instructed by the patient's physician. (See Patient's Instructions for Use.)
Drug Interactions
- Beta-Blockers: Beta-adrenergic-receptor blocking agents not only block the pulmonary effect of beta-agonists, such as Albuterol Sulfate Inhalation Aerosol, but may produce severe bronchospasm in asthmatic patients. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents in patients with asthma. In this setting, cardioselective beta-blockers should be considered, although they should be administered with caution.
- Diuretics: The ECG changes and/or hypokalemia which may result from the administration of nonpotassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonists with nonpotassium-sparing diuretics.
- Albuterol-Digoxin: Mean decreases of 16% and 22% in serum digoxin levels were demonstrated after single-dose intravenous and oral administration of albuterol, respectively, to normal volunteers who had received digoxin for 10 days. The clinical significance of these findings for patients with obstructive airway disease who are receiving albuterol and digoxin on a chronic basis is unclear; nevertheless, it would be prudent to carefully evaluate the serum digoxin levels in patients who are currently receiving digoxin and albuterol.
- Monoamine Oxidase Inhibitors or Tricyclic Antidepressants: Albuterol Sulfate Inhalation Aerosol should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, because the action of albuterol on the cardiovascular system may be potentiated.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
In a 2-year study in SPRAGUE-DAWLEY ® rats, albuterol sulfate caused a dose-related increase in the incidence of benign leiomyomas of the mesovarium at the above dietary doses of 2 mg/kg (approximately 15 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 6 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). In another study this effect was blocked by the coadministration of propranolol, a nonselective beta-adrenergic antagonist. In an 18-month study in CD-1 mice, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of up to 500 mg/kg (approximately 1700 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 800 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). In a 22-month study in Golden Hamsters, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of up to 50 mg/kg (approximately 225 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 110 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis).
Albuterol sulfate was not mutagenic in the Ames test or a mutation test in yeast. Albuterol sulfate was not clastogenic in a human peripheral lymphocyte assay or in an AH1 strain mouse micronucleus assay.
Reproduction studies in rats demonstrated no evidence of impaired fertility at oral doses up to 50 mg/kg (approximately 340 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
Pregnancy Teratogenic Effects Pregnancy
Albuterol sulfate has been shown to be teratogenic in mice. A study in CD-1 mice given albuterol sulfate subcutaneously showed cleft palate formation in 5 of 111 (4.5%) fetuses at 0.25 mg/kg (less than the maximum recommended daily inhalation dose for adults on a mg/m 2 basis) and in 10 of 108 (9.3%) fetuses at 2.5 mg/kg (approximately 8 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). The drug did not induce cleft palate formation at a dose of 0.025 mg/kg (less than the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). Cleft palate also occurred in 22 of 72 (30.5%) fetuses from females treated subcutaneously with 2.5 mg/kg of isoproterenol (positive control).
A reproduction study in Stride Dutch rabbits revealed cranioschisis in 7 of 19 (37%) fetuses when albuterol sulfate was administered orally at 50 mg/kg dose (approximately 680 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
In an inhalation reproduction study in SPRAGUE-DAWLEY rats, the albuterol sulfate/HFA-134a formulation did not exhibit any teratogenic effects at 10.5 mg/kg (approximately 70 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
A study in which pregnant rats were dosed with radiolabeled albuterol sulfate demonstrated that drug-related material is transferred from the maternal circulation to the fetus.
There are no adequate and well-controlled studies of Albuterol Sulfate Inhalation Aerosol or albuterol sulfate in pregnant women. Albuterol Sulfate Inhalation Aerosol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
During worldwide marketing experience, various congenital anomalies, including cleft palate and limb defects, have been reported in the offspring of patients being treated with albuterol. Some of the mothers were taking multiple medications during their pregnancies. Because no consistent pattern of defects can be discerned, a relationship between albuterol use and congenital anomalies has not been established.
Use in Labor and Delivery
Because of the potential for beta-agonist interference with uterine contractility, use of Albuterol Sulfate Inhalation Aerosol for relief of bronchospasm during labor should be restricted to those patients in whom the benefits clearly outweigh the risk.
Tocolysis: Albuterol has not been approved for the management of preterm labor. The benefit:risk ratio when albuterol is administered for tocolysis has not been established. Serious adverse reactions, including pulmonary edema, have been reported during or following treatment of premature labor with beta 2-agonists, including albuterol.
Nursing Mothers
Plasma levels of albuterol sulfate and HFA-134a after inhaled therapeutic doses are very low in humans, but it is not known whether the components of Albuterol Sulfate Inhalation Aerosol are excreted in human milk.
Because of the potential for tumorigenicity shown for albuterol in animal studies and lack of experience with the use of Albuterol Sulfate Inhalation Aerosol by nursing mothers, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Caution should be exercised when albuterol sulfate is administered to a nursing woman.
Pediatrics
The safety and effectiveness of Albuterol Sulfate Inhalation Aerosol in pediatric patients below the age of 4 years have not been established.
Geriatrics
Albuterol Sulfate Inhalation Aerosol has not been studied in a geriatric population. As with other beta 2-agonists, special caution should be observed when using Albuterol Sulfate Inhalation Aerosol in elderly patients who have concomitant cardiovascular disease that could be adversely affected by this class of drug.
ADVERSE REACTIONS
Adverse reaction information concerning Albuterol Sulfate Inhalation Aerosol is derived from a 12-week, double-blind, double-dummy study which compared Albuterol Sulfate Inhalation Aerosol, a CFC 11/12 propelled albuterol inhaler, and an HFA-134a placebo inhaler in 565 asthmatic patients. The following table lists the incidence of all adverse events (whether considered by the investigator drug related or unrelated to drug) from this study which occurred at a rate of 3% or greater in the Albuterol Sulfate Inhalation Aerosol treatment group and more frequently in the Albuterol Sulfate Inhalation Aerosol treatment group than in the placebo group. Overall, the incidence and nature of the adverse reactions reported for Albuterol Sulfate Inhalation Aerosol and a CFC 11/12 propelled albuterol inhaler were comparable.
|
*This table includes all adverse events (whether considered by the investigator drug related or unrelated to drug) which occurred at an incidence rate of at least 3% in the Albuterol Sulfate Inhalation Aerosol group and more frequently in the Albuterol Sulfate Inhalation Aerosol group than in the HFA-134a placebo inhaler group. |
||||
| Body System/
Adverse Event (Preferred Term) | Albuterol Sulfate Inhalation Aerosol (N=193) | CFC 11/12 Propelled Albuterol Inhaler (N=186) | HFA-134a Placebo Inhaler (N=186) | |
| Application Site Disorders | Inhalation Site Sensation | 6 | 9 | 2 |
| Inhalation Taste Sensation | 4 | 3 | 3 | |
| Body as a Whole | Allergic Reaction/Symptoms | 6 | 4 | <1 |
| Back Pain | 4 | 2 | 3 | |
| Fever | 6 | 2 | 5 | |
| Central and Peripheral Nervous System | Tremor | 7 | 8 | 2 |
| Gastrointestinal System | Nausea | 10 | 9 | 5 |
| Vomiting | 7 | 2 | 3 | |
| Heart Rate and Rhythm Disorder | Tachycardia | 7 | 2 | <1 |
| Psychiatric Disorders | Nervousness | 7 | 9 | 3 |
| Respiratory System Disorders | Respiratory Disorder
(unspecified) | 6 | 4 | 5 |
| Rhinitis | 16 | 22 | 14 | |
| Upper Resp Tract Infection | 21 | 20 | 18 | |
| Urinary System Disorder | Urinary Tract Infection | 3 | 4 | 2 |
Adverse events reported by less than 3% of the patients receiving Albuterol Sulfate Inhalation Aerosol, and by a greater proportion of Albuterol Sulfate Inhalation Aerosol patients than placebo patients, which have the potential to be related to Albuterol Sulfate Inhalation Aerosol include: dysphonia, increased sweating, dry mouth, chest pain, edema, rigors, ataxia, leg cramps, hyperkinesia, eructation, flatulence, tinnitus, diabetes mellitus, anxiety, depression, somnolence, rash. Palpitation and dizziness have also been observed with Albuterol Sulfate Inhalation Aerosol.
Adverse events reported in a 4-week pediatric clinical trial comparing Albuterol Sulfate Inhalation Aerosol and a CFC 11/12 propelled albuterol inhaler occurred at a low incidence rate and were similar to those seen in the adult trials.
In small, cumulative dose studies, tremor, nervousness, and headache appeared to be dose related.
Rare cases of urticaria, angioedema, rash, bronchospasm, and oropharyngeal edema have been reported after the use of inhaled albuterol. In addition, albuterol, like other sympathomimetic agents, can cause adverse reactions such as hypertension, angina, vertigo, central nervous system stimulation, insomnia, headache, metabolic acidosis, and drying or irritation of the oropharynx.
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc., at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms listed under ADVERSE REACTIONS, e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats per minute, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, and insomnia.
Hypokalemia may also occur. As with all sympathomimetic medications, cardiac arrest and even death may be associated with abuse of Albuterol Sulfate Inhalation Aerosol. Treatment consists of discontinuation of Albuterol Sulfate Inhalation Aerosol together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of Albuterol Sulfate Inhalation Aerosol.
The oral median lethal dose of albuterol sulfate in mice is greater than 2000 mg/kg (approximately 6800 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 3200 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). In mature rats, the subcutaneous median lethal dose of albuterol sulfate is approximately 450 mg/kg (approximately 3000 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 1400 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). In young rats, the subcutaneous median lethal dose is approximately 2000 mg/kg (approximately 14,000 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis and approximately 6400 times the maximum recommended daily inhalation dose for children on a mg/m 2 basis). The inhalation median lethal dose has not been determined in animals.
DOSAGE AND ADMINISTRATION
For treatment of acute episodes of bronchospasm or prevention of asthmatic symptoms, the usual dosage for adults and children 4 years of age and older is two inhalations repeated every 4 to 6 hours. More frequent administration or a larger number of inhalations is not recommended. In some patients, one inhalation every 4 hours may be sufficient. Each actuation of Albuterol Sulfate Inhalation Aerosol delivers 108 mcg of albuterol sulfate (equivalent to 90 mcg of albuterol base) from the mouthpiece. It is recommended to prime the inhaler before using for the first time and in cases where the inhaler has not been used for more than 2 weeks by releasing four “test sprays” into the air, away from the face.
Exercise Induced Bronchospasm Prevention: The usual dosage for adults and children 4 years of age and older is two inhalations 15 to 30 minutes before exercise.
To maintain proper use of this product, it is important that the mouthpiece be washed and dried thoroughly at least once a week. The inhaler may cease to deliver medication if not properly cleaned and dried thoroughly (see PRECAUTIONS, Information for Patients section). Keeping the plastic mouthpiece clean is very important to prevent medication buildup and blockage. The inhaler may cease to deliver medication if not properly cleaned and air dried thoroughly. If the mouthpiece becomes blocked, washing the mouthpiece will remove the blockage.
If a previously effective dose regimen fails to provide the usual response, this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
HOW SUPPLIED
Albuterol Sulfate Inhalation Aerosol is supplied as a pressurized aluminum canister, with an attached dose indicator, a yellow plastic actuator and orange dust cap each in boxes of one. Each actuation delivers 120 mcg of albuterol sulfate from the valve and 108 mcg of albuterol sulfate from the mouthpiece (equivalent to 90 mcg of albuterol base). Canisters with a labeled net weight of 6.7 g contain 200 inhalations (NDC 0781-7296-85).
Rx only. Store between 15° to 25°C (59° to 77°F). Store the inhaler with the mouthpiece down. For best results, canister should be at room temperature before use.
SHAKE WELL BEFORE USING.
The yellow actuator supplied with Albuterol Sulfate Inhalation Aerosol should not be used with any other product canisters, and actuator from other products should not be used with a Albuterol Sulfate Inhalation Aerosol canister. The correct amount of medication in each canister cannot be assured after 200 actuations and when the dose indicator display window shows zero, even though the canister is not completely empty. The canister should be discarded when the labeled number of actuations have been used.
WARNING: Avoid spraying in eyes. Contents under pressure. Do not puncture or incinerate. Exposure to temperatures above 120°F may cause bursting. Keep out of reach of children.
Albuterol Sulfate Inhalation Aerosol does not contain chlorofluorocarbons (CFCs) as the propellant.
Distributed by:
Sandoz Inc.
Princeton, NJ 08540
Copyright © 1996, 2011, 2012, 2017, 2018, 2020
All rights reserved. The trademarks depicted in this piece are owned by their respective companies.
Revised: 12/2020
INSTRUCTIONS FOR USE
Albuterol Sulfate (al-BYOO-ter-ole)
Inhalation Aerosol with Dose Indicator
Read this Instructions for Use before you start using Albuterol Sulfate Inhalation Aerosol and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment. Your doctor should show you how your child should use Albuterol Sulfate Inhalation Aerosol.
Important Information:
- Albuterol Sulfate Inhalation Aerosol is for oral inhalation use only.
- Take Albuterol Sulfate Inhalation Aerosol exactly as your doctor tells you to.
Albuterol Sulfate Inhalation Aerosol comes as a canister with a dose indicator. The dose indicator is located on the top of the canister that fits into an actuator (See Figure A). The dose indicator display window will show you how many puffs of medicine you have left. A puff of medicine is released each time you press the center of the dose indicator.
- Do not use the Albuterol Sulfate Inhalation Aerosol actuator with a canister of medicine from any other inhaler.
- Do not use the Albuterol Sulfate Inhalation Aerosol canister with an actuator from any other inhaler.
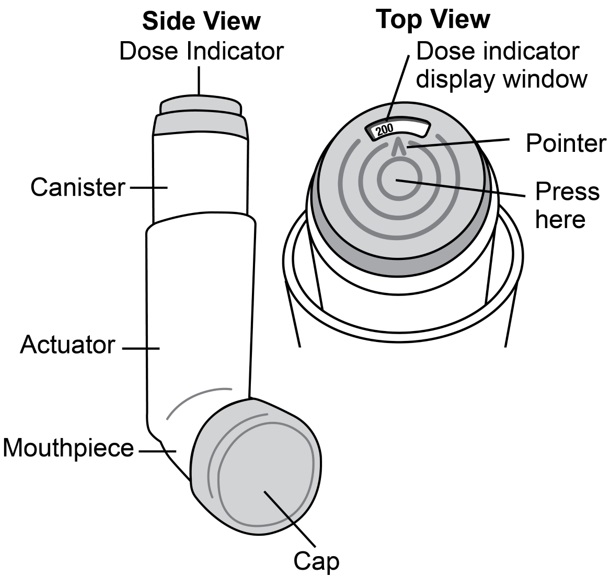
Figure A
Before you use Albuterol Sulfate Inhalation Aerosol for the first time make sure that the pointer on the dose indicator is pointing to the right of the “200” inhalation mark in the dose indicator display window (See Figure A) .
Each canister of Albuterol Sulfate Inhalation Aerosol contains 200 puffs of medicine. This does not include the sprays of medicine used for priming your inhaler.
- The dose indicator display window will continue to move after every 10 puffs.
- The number in the dose indicator display window will continue to change after every 20 puffs.
- The color in the dose indicator display window will change to red, as shown in the shaded area, when there are only 20 puffs of medicine left in your inhaler (See Figure B) . This is when you need to refill your prescription or ask your doctor if you need another prescription for Albuterol Sulfate Inhalation Aerosol.
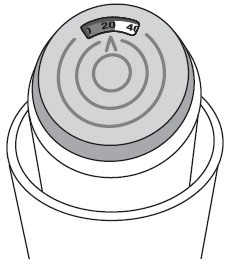
Figure B
Before using your Albuterol Sulfate Inhalation Aerosol for the first time, you should prime your inhaler. If you do not use your Albuterol Sulfate Inhalation Aerosol for more than 2 weeks, you should re-prime it before use.
- Remove the cap from the mouthpiece (See Figure C). Check inside the mouthpiece for objects before use.
- Make sure the canister is fully inserted into the actuator.
- Hold the inhaler in an upright position away from your face and shake the inhaler well.
- Press down fully on the center of the dose indicator to release a spray of medicine. You may hear a soft click from the dose indicator as it counts down during use.
- Repeat the priming step 3 more times to release a total of 4 sprays of medicine. Shake the inhaler well before each priming spray.
- After the 4 priming sprays, the dose indicator should be pointing to 200. There are now 200 puffs of medicine left in the canister.
- Your inhaler is now ready to use.
Using your Albuterol Sulfate Inhalation Aerosol inhaler:
Step 1: Shake the inhaler well before each use. Remove the cap from the mouthpiece (See Figure C). Check inside the mouthpiece for objects before use. Make sure the canister is fully inserted into the actuator.
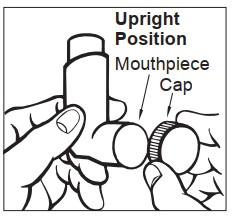
Figure C
Step 2: Breathe out as fully as you comfortably can through your mouth. Hold the inhaler in the upright position with the mouthpiece pointing towards you and place the mouthpiece fully into the mouth (See Figure D). Close your lips around the mouthpiece.
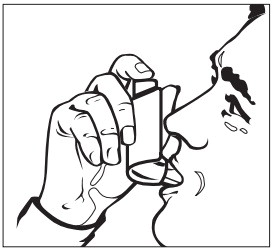
Figure D
Step 3: While breathing in deeply and slowly, press down on the center of the dose indicator with your index finger until the canister stops moving in the actuator and a puff of medicine has been released (See Figure D). Then stop pressing the dose indicator.
Step 4: Hold your breath as long as you comfortably can, up to 10 seconds. Remove the inhaler from your mouth, and then breathe out.
Step 5: If your doctor has prescribed additional puffs of Albuterol Sulfate Inhalation Aerosol, wait 1 minute then shake the inhaler well. Repeat steps 3 through 5 in the section “ Using your Albuterol Sulfate Inhalation Aerosol inhaler”.
Step 6: Replace the cap right away after use.
Cleaning your Albuterol Sulfate Inhalation Aerosol inhaler:
It is very important that you keep the mouthpiece clean so that medicine will not build up and block the spray through the mouthpiece. Clean the mouthpiece 1 time each week or if your mouthpiece becomes blocked (See Figure F).
Step 1: Remove the canister from the actuator and take the cap off the mouthpiece. Do not clean the metal canister or let it get wet.
Step 2: Wash the mouthpiece through the top and bottom with warm running water for 30 seconds (See Figure E).
Figure E
Step 3: Shake off as much water from the mouthpiece as you can.
Step 4: Look in the mouthpiece to make sure any medicine buildup has been completely washed away. If the mouthpiece is blocked with buildup, little to no medicine will come out of the mouthpiece (See Figure F). If there is any buildup, repeat Steps 2 through 4 in the section “ Cleaning your Albuterol Sulfate Inhalation Aerosol inhaler”.
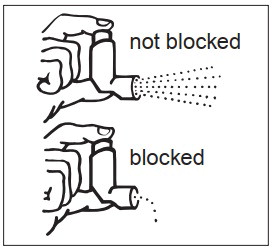
Figure F
Step 5: Let the mouthpiece air-dry such as overnight ( Figure G). Do not put the canister back into the actuator if it is still wet.
Figure G
Step 6: When the mouthpiece is dry, put the canister back in the actuator and put the cap on the mouthpiece.
Note: If you need to use your Albuterol Sulfate Inhalation Aerosol inhaler before it is completely dry, put the canister back in the actuator and shake the inhaler well. Press down on the center of the dose indicator 2 times to release a total of 2 sprays into the air, away from your face. Take your dose as prescribed then clean and air-dry your inhaler as described in the section “ Cleaning your Albuterol Sulfate Inhalation Aerosol inhaler”.
How should I store Albuterol Sulfate Inhalation Aerosol?
- Store Albuterol Sulfate Inhalation Aerosol at room temperature between 59°F and 77°F (15°C and 25°C).
- Store with the mouthpiece down.
- Avoid exposing Albuterol Sulfate Inhalation Aerosol to extreme heat and cold.
- Do not puncture or burn the canister.
- Keep your Albuterol Sulfate Inhalation Aerosol inhaler and all medicines out of the reach of children.
Developed and Manufactured by:
Kindeva Drug Delivery L.P.
Northridge, CA 91324, USA
Distributed by:
Sandoz Inc.
Princeton, NJ 08540
Copyright © 1996, 2011, 2012, 2017, 2018, 2020All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 12/2020
NITROGLYCERIN TABLETS
These highlights do not include all the information needed to use NITROGLYCERIN SUBLINGUAL TABLETS safely and effectively. See full prescribing information for NITROGLYCERIN SUBLINGUAL TABLETS
NITROGLYCERIN sublingual tablets, for oral use
Initial U.S. Approval: 1981
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Nitroglycerin sublingual tablets are indicated for the acute relief of an attack or acute prophylaxis of angina pectoris due to coronary artery disease.
2 DOSAGE AND ADMINISTRATION
Administer one tablet under the tongue or in the buccal pouch at the first sign of an acute anginal attack. Allow tablet to dissolve without swallowing. One additional tablet may be administered every 5 minutes until relief is obtained. No more than three tablets are recommended within a 15-minute period. If the pain persists after a total of 3 tablets in a 15-minute period, or if the pain is different than is typically experienced, seek prompt medical attention.
Nitroglycerin sublingual tablets may be used prophylactically 5 to 10 minutes prior to engaging in activities that might precipitate an acute attack.
For patients with xerostomia, a small sip of water prior to placing the tablet under the tongue may help maintain mucosal hydration and aid dissolution of the tablet.
Administer nitroglycerin sublingual tablets at rest, preferably in the sitting position.
3 DOSAGE FORMS AND STRENGTHS
Nitroglycerin Sublingual Tablets, USP are supplied as white to off-white, round, flat-faced tablets in three strengths:
0.3 mg (Debossed with “1” on one side and “C” on the other)
0.4 mg (Debossed with “2” on one side and “C” on the other)
0.6 mg (Debossed with “3” on one side and “C” on the other)
4 CONTRAINDICATIONS
4.1 PDE-5-Inhibitors and sGC-Stimulators
Do not use nitroglycerin sublingual tablets in patients who are taking PDE-5 Inhibitors, such as avanafil, sildenafil, tadalafil, vardenafil hydrochloride. Concomitant use can cause severe hypotension, syncope, or myocardial ischemia [see Drug Interactions (7.1)].
Do not use nitroglycerin sublingual tablets in patients who are taking the soluble guanylate cyclase stimulators, such as riociguat. Concomitant use can cause hypotension.
4.2 Severe Anemia
Nitroglycerin sublingual tablets are contraindicated in patients with severe anemia (large doses of nitroglycerin may cause oxidation of hemoglobin to methemoglobin and could exacerbate anemia).
4.3 Increased Intracranial Pressure
Nitroglycerin sublingual tablets may precipitate or aggravate increased intracranial pressure and thus should not be used in patients with possible increased intracranial pressure (e.g., cerebral hemorrhage or traumatic brain injury).
5 WARNINGS AND PRECAUTIONS
- Tolerance: Excessive use may lead to tolerance. (5.1)
- Hypotension: Severe hypotension may occur. (5.2)
5.1 Tolerance
Excessive use may lead to the development of tolerance. Only the smallest dose required for effective relief of the acute angina attack should be used. A decrease in therapeutic effect of sublingual nitroglycerin may result from use of long-acting nitrates.
5.2 Hypotension
Severe hypotension, particularly with upright posture, may occur with small doses of nitroglycerin particularly in patients with constrictive pericarditis, aortic or mitral stenosis, patients who may be volume-depleted, or are already hypotensive. Hypotension induced by nitroglycerin may be accompanied by paradoxical bradycardia and increased angina pectoris. Symptoms of severe hypotension (nausea, vomiting, weakness, pallor, perspiration and collapse/syncope) may occur even with therapeutic doses.
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail elsewhere in the label:
Hypotension [see Warnings and Precautions (5.2)]
Headache [see Warnings and Precautions (5.4)]
Hypersensitivity [see Contraindications (4.4)]
Vertigo, dizziness, weakness, palpitation, and other manifestations of postural hypotension may develop occasionally, particularly in erect, immobile patients. Marked sensitivity to the hypotensive effects of nitrates (manifested by nausea, vomiting, weakness, diaphoresis, pallor, and collapse) may occur at therapeutic doses. Syncope due to nitrate vasodilatation has been reported.
Flushing, drug rash, and exfoliative dermatitis have been reported in patients receiving nitrate therapy.
7 DRUG INTERACTIONS
Ergotamine: increased bioavailability of ergotamine. Avoid concomitant use. (7.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2021
7.1 PDE-5-Inhibitors and sGC-Stimulators
Nitroglycerin sublingual tablets are contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE-5). PDE-5-Inhibitors such as avanafil, sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates.
Nitroglycerin sublingual tablets are contraindicated in patients who are taking soluble guanylate cyclase (sGC) stimulators. Concomitant use can cause hypotension.
The time course and dose dependence of these interactions have not been studied, and use within a few days of one another is not recommended. Appropriate supportive care for the severe hypotension has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.
7.2 Ergotamine
Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and subsequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore, patients receiving sublingual nitroglycerin should avoid ergotamine and related drugs or be monitored for symptoms of ergotism if this is not possible.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published data on the use of nitroglycerin are insufficient to determine a drug associated risk of major birth defects or miscarriage. In animal reproduction studies, there were no adverse developmental effects when nitroglycerin was administered intravenously to rabbits or intraperitoneally to rats during organogenesis at doses greater than 64-times the human dose [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
No embryotoxic or postnatal development effects were observed with transdermal application in pregnant rabbits and rats at doses up to 80 and 240 mg/kg/day, respectively, at intraperitoneal doses in pregnant rats up to 20 mg/kg/day from gestation day 7 to 17, and at intravenous doses in pregnant rabbits up to 4 mg/kg/day from gestation day 6 to 18.
8.4 Pediatric Use
The safety and effectiveness of nitroglycerin in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of nitroglycerin sublingual tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
10.1 Signs and Symptoms, Methemoglobinemia
Nitrate overdosage may result in: severe hypotension, persistent throbbing headache, vertigo, palpitation, visual disturbance, flushing and perspiring skin (later becoming cold and cyanotic), nausea and vomiting (possibly with colic and even bloody diarrhea), syncope (especially in the upright posture), methemoglobinemia with cyanosis and anorexia, initial hyperpnea, dyspnea and slow breathing, slow pulse (dicrotic and intermittent), heart block, increased intracranial pressure with cerebral symptoms of confusion and moderate fever, paralysis and coma followed by clonic convulsions, and possibly death due to circulatory collapse.
Case reports of clinically significant methemoglobinemia are rare at conventional doses of organic nitrates. The formation of methemoglobin is dose-related and in the case of genetic abnormalities of hemoglobin that favor methemoglobin formation, even conventional doses of organic nitrates could produce harmful concentrations of methemoglobin.
10.2 Treatment of Overdosage
As hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. No specific antagonist to the vasodilator effects of nitroglycerin is known. Keep the patient recumbent in a shock position and comfortably warm. Passive movement of the extremities may aid venous return. Intravenous infusion of normal saline or similar fluid may also be necessary. Administer oxygen and artificial ventilation, if necessary. If methemoglobinemia is present, administration of methylene blue (1% solution), 1 to 2 mg per kilogram of body weight intravenously, may be required unless the patient is known to have G-6-PD deficiency. If an excessive quantity of nitroglycerin has been recently swallowed gastric lavage may be of use.
As epinephrine is ineffective in reversing the severe hypotensive events associated with overdosage, it is not recommended for resuscitation.
11 DESCRIPTION
Nitroglycerin Sublingual Tablets, USP are stabilized sublingual compressed nitroglycerin tablet that contains 0.3 mg, 0.4 mg, or 0.6 mg nitroglycerin; as well as calcium stearate, croscarmellose sodium, silica dimethyl silylate and lactose monohydrate.
Nitroglycerin, an organic nitrate, is a vasodilating agent. The chemical name for nitroglycerin is 1, 2, 3 propanetriol trinitrate and the chemical structure is:
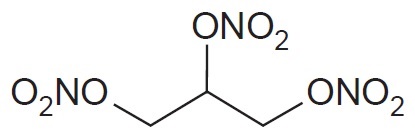
Molecular weight: 227.09 g/mol
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nitroglycerin forms free radical nitric oxide (NO) which activates guanylate cyclase, resulting in an increase of guanosine 3'5' monophosphate (cyclic GMP) in smooth muscle and other tissues. These events lead to dephosphorylation of myosin light chains, which regulate the contractile state in smooth muscle, and result in vasodilatation.
12.2 Pharmacodynamics
The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle. Although venous effects predominate, nitroglycerin produces, in a dose-related manner, dilation of both arterial and venous beds. Dilation of postcapillary vessels, including large veins, promotes peripheral pooling of blood, decreases venous return to the heart, and reduces left ventricular end-diastolic pressure (preload).
Nitroglycerin also produces arteriolar relaxation, thereby reducing peripheral vascular resistance and arterial pressure (afterload), and dilates large epicardial coronary arteries; however, the extent to which this latter effect contributes to the relief of exertional angina is unclear.
Therapeutic doses of nitroglycerin may reduce systolic, diastolic, and mean arterial blood pressure. Effective coronary perfusion pressure is usually maintained, but can be compromised if blood pressure falls excessively, or increased heart rate decreases diastolic filling time.
Elevated central venous and pulmonary capillary wedge pressures, and pulmonary and systemic vascular resistance are also reduced by nitroglycerin therapy. Heart rate is usually slightly increased, presumably due to a compensatory response to the fall in blood pressure. Cardiac index may be increased, decreased, or unchanged. Myocardial oxygen consumption or demand (as measured by the pressure-rate product, tension-time index, and stroke-work index) is decreased and a more favorable supply-demand ratio can be achieved. Patients with elevated left ventricular filling pressures and increased systemic vascular resistance in association with a depressed cardiac index are likely to experience an improvement in cardiac index. In contrast, when filling pressures and cardiac index are normal, cardiac index may be slightly reduced following nitroglycerin administration.
Consistent with the symptomatic relief of angina, digital plethysmography indicates that onset of the vasodilatory effect occurs approximately 1 to 3 minutes after sublingual nitroglycerin administration and reaches a maximum by 5 minutes postdose. Effects persist for at least 25 minutes following nitroglycerin administration.
12.3 Pharmacokinetics
Absorption
Nitroglycerin is rapidly absorbed following sublingual administration of nitroglycerin sublingual tablets. Mean peak nitroglycerin plasma concentrations occur at a mean time of approximately 6 to 7 minutes postdose (Table 1). Maximum plasma nitroglycerin concentrations (Cmax) and area under the plasma concentration-time curves (AUC) increase dose-proportionally following 0.3 to 0.6 mg nitroglycerin. The absolute bioavailability of nitroglycerin from nitroglycerin sublingual tablets is approximately 40% but tends to be variable due to factors influencing drug absorption, such as sublingual hydration and mucosal metabolism.
| Mean Nitroglycerin (SD) Values | ||
| 2 x 0.3 mg | 1 x 0.6 mg | |
| Parameter | Nitroglycerin Sublingual Tablets | Nitroglycerin Sublingual Tablets |
| Cmax, ng/mL | 2.3 (1.7) | 2.1 (1.5) |
| Tmax, min | 6.4 (2.5) | 7.2 (3.2) |
| AUC (0-∞), min | 14.9 (8.2) | 14.9 (11.4) |
| t½, min | 2.8 (1.1) | 2.6 (0.6) |
Distribution
The volume of distribution (VArea) of nitroglycerin following intravenous administration is 3.3 L/kg. At plasma concentrations between 50 and 500 ng/mL, the binding of nitroglycerin to plasma proteins is approximately 60%, while that of 1,2- and 1,3-dinitroglycerin is 60% and 30%, respectively.
Metabolism
A liver reductase enzyme is of primary importance in the metabolism of nitroglycerin to glycerol di- and mononitrate metabolites and ultimately to glycerol and organic nitrate. Known sites of extrahepatic metabolism include red blood cells and vascular walls. In addition to nitroglycerin, 2 major metabolites 1,2- and 1,3-dinitroglycerin, are found in plasma. Mean peak 1,2- and 1,3-dinitroglycerin plasma concentrations occur at approximately 15 minutes postdose. The elimination half-life of 1,2- and 1,3-dinitroglycerin is 36 and 32 minutes, respectively. The 1,2- and 1,3-dinitroglycerin metabolites have been reported to possess approximately 2% and 10%, respectively, of the pharmacological activity of nitroglycerin. Higher plasma concentrations of the dinitro metabolites, along with their nearly 10-fold longer elimination half-lives, may contribute significantly to the duration of pharmacologic effect. Glycerol mononitrate metabolites of nitroglycerin are biologically inactive.
Elimination
Nitroglycerin plasma concentrations decrease rapidly, with a mean elimination half-life of 2 to 3 minutes. Half-life values range from 1.5 to 7.5 minutes. Clearance (13.6 L/min) greatly exceeds hepatic blood flow. Metabolism is the primary route of drug elimination.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal carcinogenesis studies with sublingually administered nitroglycerin have not been performed.
Carcinogenicity potential of nitroglycerin was evaluated in rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years. Rats developed dose-related fibrotic and neoplastic changes in liver, including carcinomas, and interstitial cell tumors in testes. At high dose, the incidences of hepatocellular carcinomas in males was 48% and in females was 33%, compared to 0% in untreated controls. Incidences of testicular tumors were 52% vs. 8% in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice.
Nitroglycerin was mutagenic in Ames tests performed in 2 different laboratories. Nevertheless, there was no evidence of mutagenicity in an in vivo dominant lethal assay with male rats treated with doses up to about 363 mg/kg/day, PO, or in ex vivo cytogenetic tests in rat and dog cells.
In a 3-generation reproduction study, rats received dietary nitroglycerin at doses up to about 434 mg/kg/day for 6 months prior to mating of the F0 generation, with treatment continuing through successive F1 and F2 generations. The high dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males. In this 3-generation study, there was no clear evidence of teratogenicity.
16 HOW SUPPLIED/STORAGE AND HANDLING
Nitroglycerin Sublingual Tablets, USP are supplied as white to off white, round, flat-faced tablets in 3 strengths (0.3 mg, 0.4 mg, and 0.6 mg) in bottles.
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature]. Nitroglycerin should be kept in the original glass container and must be tightly capped after each use to prevent loss of tablet potency.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
This product's label may have been updated. For full prescribing information, please visit www.glenmarkpharma-us.com.
Manufactured by:
Glenmark Pharmaceuticals Limited
India
Manufactured for:

Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
October 2021
Patient Information
Nitroglycerin (nahy-truh-glis-er-in)
Sublingual Tablets, USP
Read this information carefully before you start nitroglycerin sublingual tablets and each time you refill your prescription. There may be new information. This information does not replace talking with your doctor. If you have any questions about nitroglycerin sublingual tablets, ask your doctor. Your doctor will know if nitroglycerin sublingual tablets are right for you.
What are Nitroglycerin Sublingual Tablets?
Nitroglycerin sublingual tablets are type of medicine known as an organic nitrate and is a vasodilating agent. It is used to treat a type of chest pain called angina.
What is Angina?
Angina is a pain or discomfort that keeps coming back when part of your heart does not get enough blood. Angina feels like a pressing or squeezing pain, usually in your chest under the breastbone. Sometimes you can feel it in your shoulders, arms, neck, jaws, or back. Nitroglycerin sublingual tablets can relieve this pain.
Who should not use Nitroglycerin Sublingual Tablets?
Do not use nitroglycerin sublingual tablets if you are allergic to organic nitrates (like the active ingredient in nitroglycerin).
You should not take nitroglycerin sublingual tablets if you have the following conditions:
- very recent heart attack
- severe anemia
- increased pressure in the head
Do not take nitroglycerin sublingual tablets with drugs for erectile dysfunction, like VIAGRA® (sildenafil citrate), CIALIS® (tadalafil), or LEVITRA® (vardenafil hydrochloride), as this may lead to extreme lowering of your blood pressure.
Do not take nitroglycerin sublingual tablets if you take medicines called guanylate cyclase stimulators which include riociguat, a medicine that treats pulmonary arterial hypertension and chronic-thromboembolic pulmonary hypertension.
What should I tell my doctor before taking Nitroglycerin Sublingual Tablets?
Before using nitroglycerin sublingual tablets, tell your doctor if:
- You are taking any medicines that are used to treat angina, heart failure, or an irregular heartbeat.
- You are taking any medicines that reduce blood pressure.
- You are taking any diuretics (water pills).
- You are taking medicines that can cause dry mouth such as tricyclic antidepressants (e.g. amitriptyline, desipramine, doxepin), anticholinergic drugs, or any antimuscarinic drugs (e.g. atropine).
- You are taking ergotamine or similar drugs for migraine headaches.
- You are taking aspirin.
- You are taking any medicines for erectile dysfunction.
- You are pregnant or plan to become pregnant.
- You are breastfeeding.
How should I take Nitroglycerin Sublingual Tablets?
- Do not chew, crush, or swallow nitroglycerin sublingual tablets.
- You should sit down when taking nitroglycerin sublingual tablets and use caution when you stand up. This eliminates the possibility of falling due to lightheadedness or dizziness.
- One tablet should be dissolved under the tongue or in the oral cavity at the first sign of chest pain.
- The dose may be repeated approximately every 5 minutes, until the chest pain is relieved.
- If the pain persists after a total of 3 tablets in a 15-minute period, or is different than you typically experience, call your doctor or seek emergency help.
- Nitroglycerin sublingual tablets may be used 5 to 10 minutes prior to activities that might cause chest pain.
- You may feel a burning or tingling sensation in your mouth when you take nitroglycerin sublingual tablets.
What should I avoid while taking Nitroglycerin Sublingual Tablets?
- Do not breastfeed. It is not known if nitroglycerin will pass through your milk.
- Do not consume alcohol while taking nitroglycerin sublingual tablets, as this can lower your blood pressure.
- Do not start any new prescription or non-prescription medicines or supplements, unless you check with your doctor first.
What are the possible side effects of Nitroglycerin Sublingual Tablets?
Nitroglycerin sublingual tablets may cause the following side effects:
- headache
- vertigo (a major symptom of balance disorder)
- dizziness
- weakness
- heart palpitations (unusual awareness of the heartbeat)
- low blood pressure upon rising from a seated position
- nausea and vomiting
- sweating
- paleness
- fainting
- flushing (warm or red condition of your skin)
- other skin reactions that may be severe
Tell your doctor if you are concerned about any side effects you experience. These are not all the possible side effects of nitroglycerin sublingual tablets. For a complete list, ask your doctor or pharmacist.
How do I store Nitroglycerin Sublingual Tablets?
Nitroglycerin sublingual tablets should be kept in the original glass container and tightly capped after each use to prevent loss of tablet potency.
Store nitroglycerin sublingual tablets at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature].
General advice about Nitroglycerin Sublingual Tablets
Sometimes doctors will prescribe a medicine for a condition that is not included in the patient information leaflets. Only use nitroglycerin sublingual tablets the way your doctor told you to. Do not give nitroglycerin sublingual tablets to other people, even if they have the same symptoms you have. They may harm them.
You can ask your pharmacist or doctor for information about nitroglycerin sublingual tablets, or you can call Glenmark Pharmaceuticals Inc., USA at 1 (888) 721-7115.
Trademarks are the property of their respective owners.
Manufactured by:
Glenmark Pharmaceuticals Limited
India
Manufactured for:

Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
October 2021
DESCRIPTION
Diphenhydramine Hydrochloride Injection is a sterile, nonpyrogenic solution for intravenous or deep intramuscular use as an antihistaminic agent. Each mL contains diphenhydramine hydrochloride 50 mg and benzethonium chloride 100 mcg in Water for Injection. pH 4.0-6.5; sodium hydroxide and/or hydrochloric acid added, if needed, for pH adjustment.
The chemical name of diphenhydramine hydrochloride is 2-(Diphenylmethoxy)-N,N-dimethylethylamine hydrochloride. The structural formula is as follows:
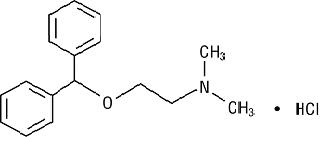
C17H21NO • HCl MW 291.82
Diphenhydramine hydrochloride occurs as a white crystalline powder and is freely soluble in water and alcohol.
CLINICAL PHARMACOLOGY
Diphenhydramine hydrochloride is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector cells.
Diphenhydramine hydrochloride in the injectable form has a rapid onset of action. Diphenhydramine is widely distributed throughout the body, including the CNS. A portion of the drug is excreted unchanged in the urine, while the rest is metabolized via the liver. Detailed information on the pharmacokinetics of Diphenhydramine Hydrochloride Injection is not available.
INDICATIONS AND USAGE
Diphenhydramine Hydrochloride Injection is effective in adults and pediatric patients, other than premature infants and neonates, for the following conditions when the oral form is impractical:
Antihistaminic
For amelioration of allergic reactions to blood or plasma, in anaphylaxis as an adjunct to epinephrine and other standard measures after the acute symptoms have been controlled and for other uncomplicated allergic conditions of the immediate type when oral therapy is impossible or contraindicated.
Antiparkinsonism
For use in parkinsonism, when oral therapy is impossible or contraindicated, as follows: parkinsonism in the elderly who are unable to tolerate more potent agents, mild cases of parkinsonism in other age groups and in other cases of parkinsonism in combination with centrally acting anticholinergic agents.
CONTRAINDICATIONS
Use in Nursing Mothers
Because of the higher risk of antihistamines for infants generally, and for neonates and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
WARNINGS
Antihistamines should be used with considerable caution in patients with narrow-angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, symptomatic prostatic hypertrophy or bladder-neck obstruction.
Local necrosis has been associated with the use of subcutaneous or intradermal use of intravenous diphenhydramine.
PRECAUTIONS
General
Diphenhydramine hydrochloride has an atropine-like action and, therefore, should be used with caution in patients with a history of bronchial asthma, increased intraocular pressure, hyperthyroidism, cardiovascular disease or hypertension. Use with caution in patients with lower respiratory disease, including asthma.
Information for Patients
Patients taking diphenhydramine hydrochloride should be advised that this drug may cause drowsiness and has an additive effect with alcohol.
Patients should be warned about engaging in activities requiring mental alertness, such as driving a car or operating appliances, machinery, etc.
Drug Interactions
Diphenhydramine hydrochloride has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.)
MAO inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to determine mutagenic and carcinogenic potential have not been performed.
Pregnancy
Teratogenic Effects—Pregnancy Category B
Reproduction studies have been performed in rats and rabbits at doses up to 5 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to diphenhydramine hydrochloride. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pediatric Use
Diphenhydramine should not be used in neonates and premature infants (see CONTRAINDICATIONS).
Diphenhydramine may diminish mental alertness, or in the young pediatric patient, cause excitation. Overdosage may cause hallucinations, convulsions or death (see WARNINGS and OVERDOSAGE).
See also DOSAGE AND ADMINISTRATION section.
ADVERSE REACTIONS
The most frequent adverse reactions are italicized.
General
Urticaria; drug rash; anaphylactic shock; photosensitivity; excessive perspiration; chills; dryness of mouth, nose and throat.
OVERDOSAGE
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in pediatric patients. Atropine-like signs and symptoms, dry mouth; fixed, dilated pupils; flushing, and gastrointestinal symptoms may also occur.
Stimulants should not be used.
Vasopressors may be used to treat hypotension.
DOSAGE AND ADMINISTRATION
THIS PRODUCT IS FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION ONLY.
Diphenhydramine Hydrochloride Injection is indicated when the oral form is impractical.
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
Pediatric Patients, Other Than Premature Infants and Neonates
5 mg/kg/24 hours or 150 mg/m2/24 hours. Maximum daily dosage is 300 mg. Divide into four doses, administered intravenously at a rate generally not exceeding 25 mg/min, or deep intramuscularly.
Adults
10 to 50 mg intravenously at a rate generally not exceeding 25 mg/min, or deep intramuscularly; 100 mg if required; maximum daily dosage is 400 mg.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
Diphenhydramine Hydrochloride Injection, USP 50 mg/mL
Storage
Protect from light. Keep covered in carton until time of use. Store at 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689, or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
For Product Inquiry call 1-877-845-0689.
Manufactured by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
Revised December 2019
Uses
- temporarily relieves minor aches and pains due to:
- headache
- muscle pain
- toothache
- menstrual pain
- colds
- minor pain of arthritis
- temporarily reduces fever
Warnings
Reye's syndrome:
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert:
Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning:
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you are allergic to aspirin or any other pain reliever/fever reducer
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if you are taking a prescription drug for
- gout
- diabetes
- arthritis
Stop use and ask doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- ringing in the ears or a loss of hearing occurs
If pregnant or breast-feeding, ask a health professional before use.
It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Directions
- drink a full glass of water with each dose
- adults and children 12 years and over: take 1 or 2 tablets every 4 hours or 3 tablets every 6 hours, not to exceed 12 tablets in 24 hours
- children under 12 years: consult a doctor
This product is repackaged by: Select Corporation
1433 Wainwright Way, Carrollton, TX 75007
877-244-4400 M - F 9am - 5 pm, from a product manufactured by Bayer.
Dist. by: Bayer HealthCare LLC
Whippany, NJ 07981
TRUEplus GLUCOSE TABLETS
15 g Fast acting Carbohydrates per Serving
Raises low blood sugar and boosts energy
Fat Free
Gluten Free
Sodium Free
ORANGE NATURALLY & ARTIFICIALLY FLAVORED
DO NOT USE IF PROTECTIVE SEAL IS BROKEN OR MISSING
QUESTIONS? Call 1-800-803-6025
INGREDIENTS: DEXTROSE (D-GLUCOSE), MALTODEXTRIN, CITRIC ACID, MAGNESIUM STEARATE, MICROCRYSTALLINE CELLUOSE, NATURAL & ARTIFICIAL ORANGE FLAVOR, ASCORBIC ACID, YELLOW #6.
DIRECTIONS
Chew desired amount of glucose.
Store at room temperature. Do not refrigerate or freeze. Do not expose to excessive heat or moisture.
Distributed by McKesson
6555, State Highway 161,
Las Colinas, TX 75039
TRUEplus is a trademark of Trivida Health, Inc.
healthmart.com
KIT CONTAINS:
EPINEPHRINE AUTO-INJECT (0.3 mg) – 1 no.
ALBUTEROL SULFATE HFA INHALER (90 mcg) – 1 no.
NITROGLYCERIN SUBLINGUAL TABLETS (0.4 mg) – 1 bottle (25 tablets)
DIPHENHYDRAMINE HCl INJECTION SOLUTION (50 mg/mL) – 1 vial
ASPIRIN TABLETS (325 mg) – 1 Pouch (2 nos).
GLUCOSE TABLETS – 1 bottle (10 nos).
BD PLASTIPAK 3 mL SYRINGE 25GX1-1/2" – 1 no.
BESTDENTAL.COM

