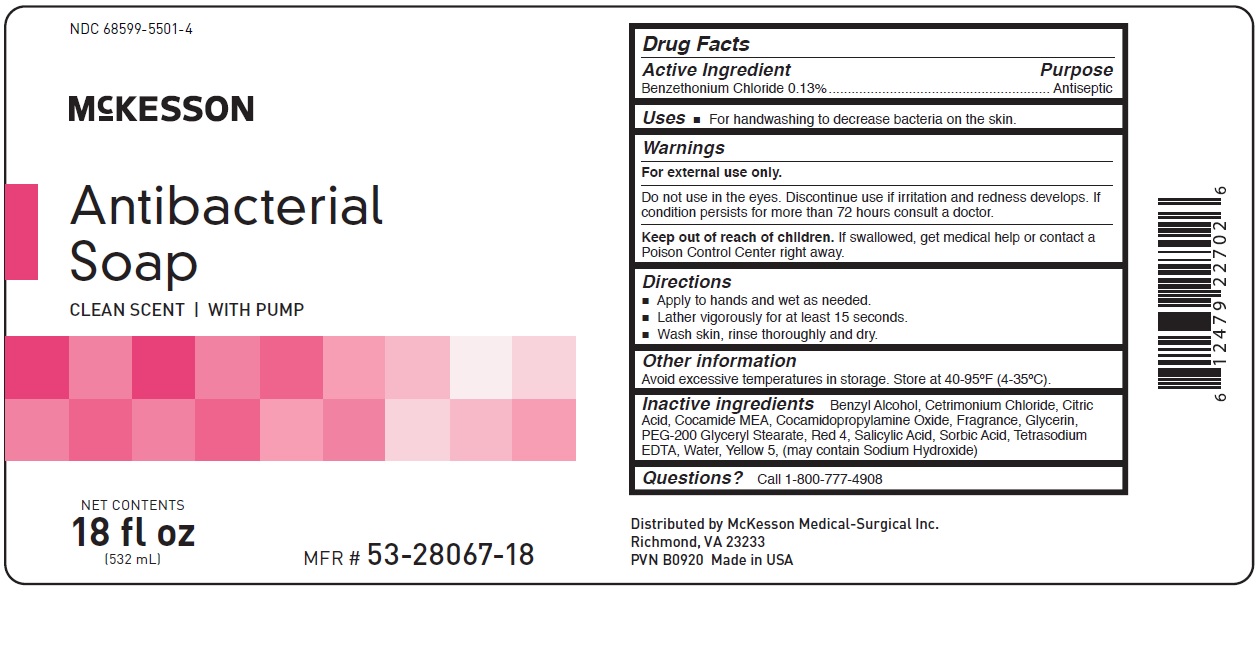
Warnings
For external use only
.
Do not use in the eyes. Discontinue use if irritation and redness
develops. If condition persists for more than 72 hours consult a
doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
• Apply to hands and wet as needed.
• Lather vigorously for at least 15 seconds.
• Wash skin, rinse thoroughly and dry.
Inactive Ingredients:
Benzyl Alcohol, Cetrimonium Chloride, Citric Acid, Cocamide MEA, Cocamidopropylamine Oxide, Fragrance,
Glycerin, PEG-200 Glyceryl Stearate, Red 4, Salicylic Acid, Sorbic Acid, Tetrasodium EDTA, Water, Yellow 5,
(may contain Sodium Hydroxide)


 NDC 68599-5501-2
NDC 68599-5501-2