Active ingredient
Benzalkonium Chloride 0.13%
Uses
- for hand sanitizing to decrease bacteria on the skin
- recommended for repeated use
Warnings
For external use only
When using this product avoid contact with eyes. In case of eye contact, flush eyes with water.
Stop use and ask a doctor if irritation or redness develop
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- take wipe and rub thoroughly over all surfaces of both hands
- rub hands together briskly to dry
- dispose of wipe
Inactive ingredients Purified Water, Glycerin, Aloe Barbadensis Leaf Juice, Decyl Glucoside
Manufactured for Terraboost Media, LLC
3109 Grand Avenue #300
Miami, FL 33133
877-837-7210
A Product of Terraboost®
877-837-7210
www.tazzabrands.com
Cannister
NDC 76370-0001-3
tazza®
HAND SANITIZING WIPES
Alcohol-Free Formula
Contains Benzalkonium Chloride
Kills 99.99% of most common germs
1200 Wet Wipes
4.3" x 7.1" ((11cm x 18cm)

Pouch
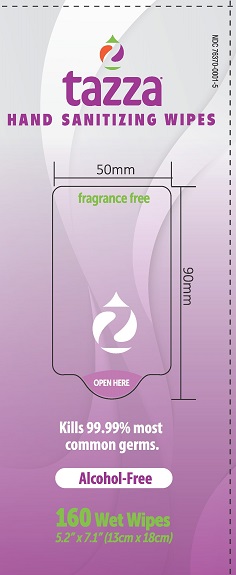
NDC 76370-0001-5
tazza®
HAND SANITIZING WIPES
fragrance free
Kills 99.99% most common germs.
Alcohol-Free
160 Wet Wipes
5.2 X 7.1" (13cm X 18cm)
Tazza Brands East Inc.

