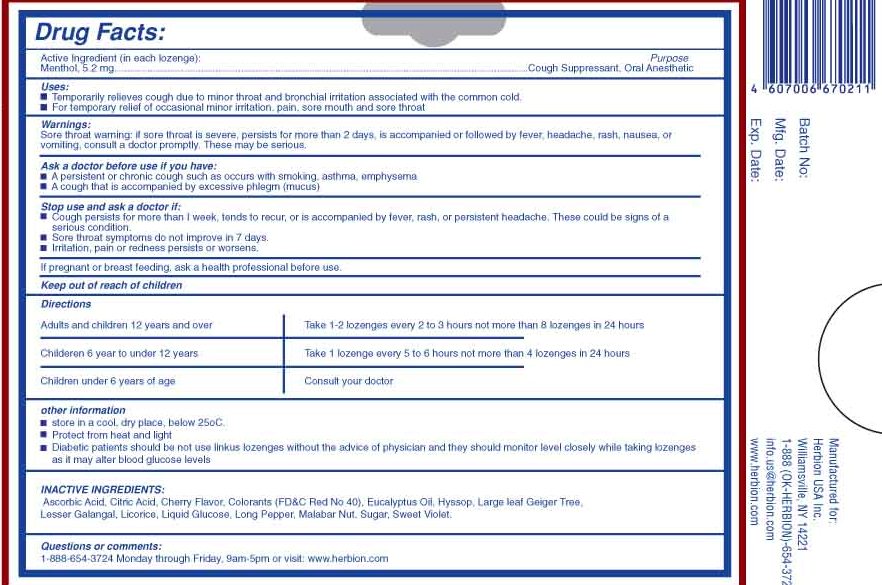
Uses:
- Temporarily relieves cough due to minor throat and bronchial irritation associated with the common cold
- For temporary relief of occasional minor irritation, pain, sore mouth and sore throat
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomitting, consult a doctor promptly. These may be serious.
Stop use and ask a doctor if:
- Cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persisted headache. These could be signs of a serious condition
- Sore throat symptoms do not improve in 7 days
- Irritation, pain or redness persists or worsens
Ask a doctor before use if you have:
- A persistent or chronic cough such as occurs with smoking, asthma, emphysema
- A cough that is accompanied by excessive phlegm (mucus)
Directions
Adults and children 12 years and over Take 1-2 lozenges every 2 to 3 hours not more than 8 lozenges in 24 hours
Children 6 years to under 12 years Take 1 lozenge every 5 to 6 hours not more than 4 lozenges in 24 hours
Children under 6 years of age Consult your doctor
Other Information:
- store in a cool, dry place, below 25 degrees C
- Protect from heat and light
- Diabetic patients should not use linkus lozenges without the advice of physician and they should monitor glucose levels closely while taking lozenges as it may alter blood glucose levels
Manufactured for:
Herbion USA Inc.
Williamsville NY 14221
1-888-(OK-HERBION)-654-3724
info.us@herbion.com
www.herbion.com