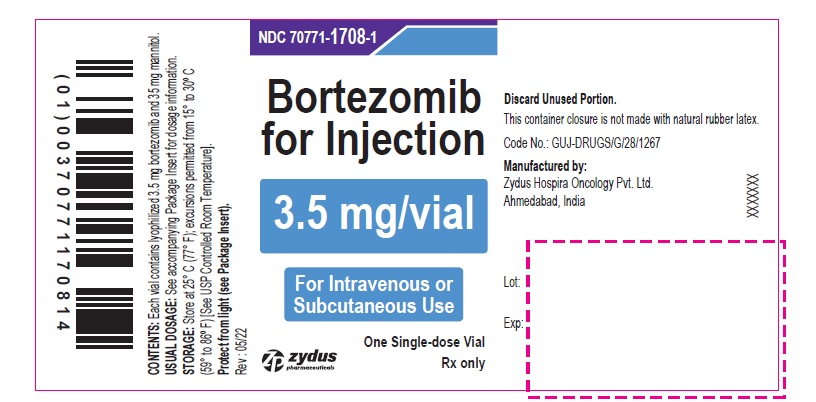
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Bortezomib for Injection
3.5 mg/vial
For Intravenous or Subcutaneous Use
One Single-dose Vial
Rx only
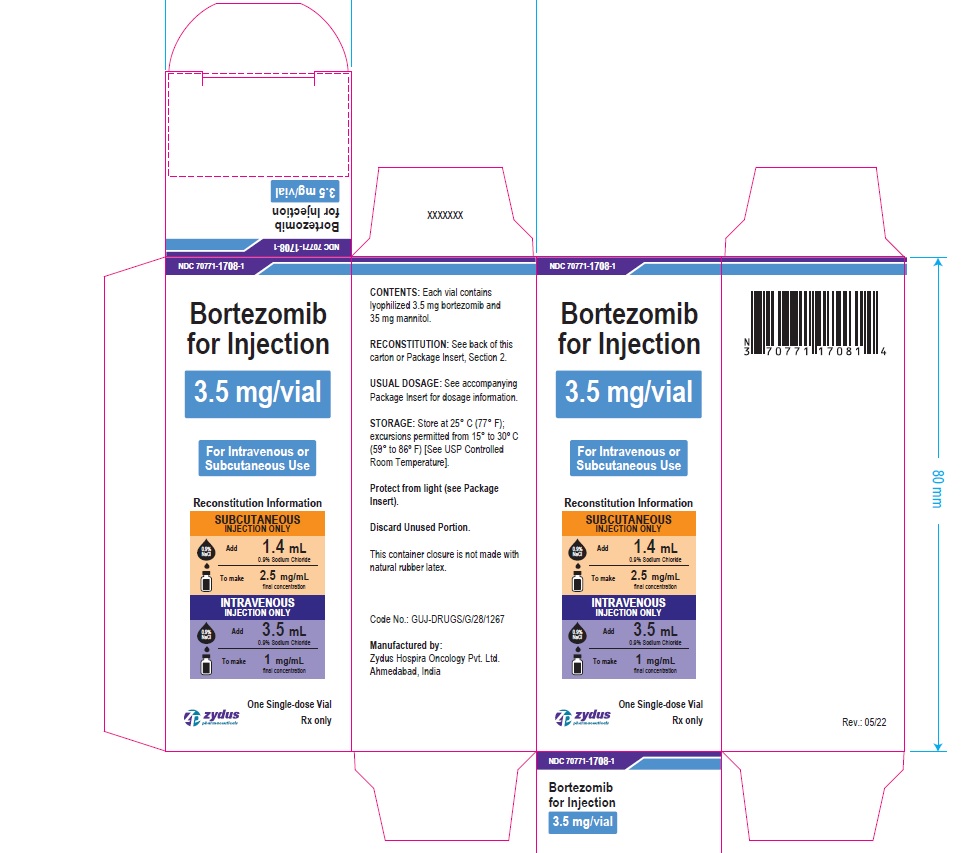
Bortezomib for Injection – Carton label
3.5 mg/vial
For Intravenous or Subcutaneous Use
Reconstitution Information
SUBCUTANEOUS INJECTION ONLY
0.9% NaCl
Add
1.4 mL
0.9% Sodium Chloride
To make
2.5 mg/mL
final concentration
INTRAVENOUS INJECTION ONLY
0.9% NaCl
Add
3.5 mL
0.9% Sodium Chloride
To make
1 mg/mL
final concentration
One Single-dose Vial
Rx only
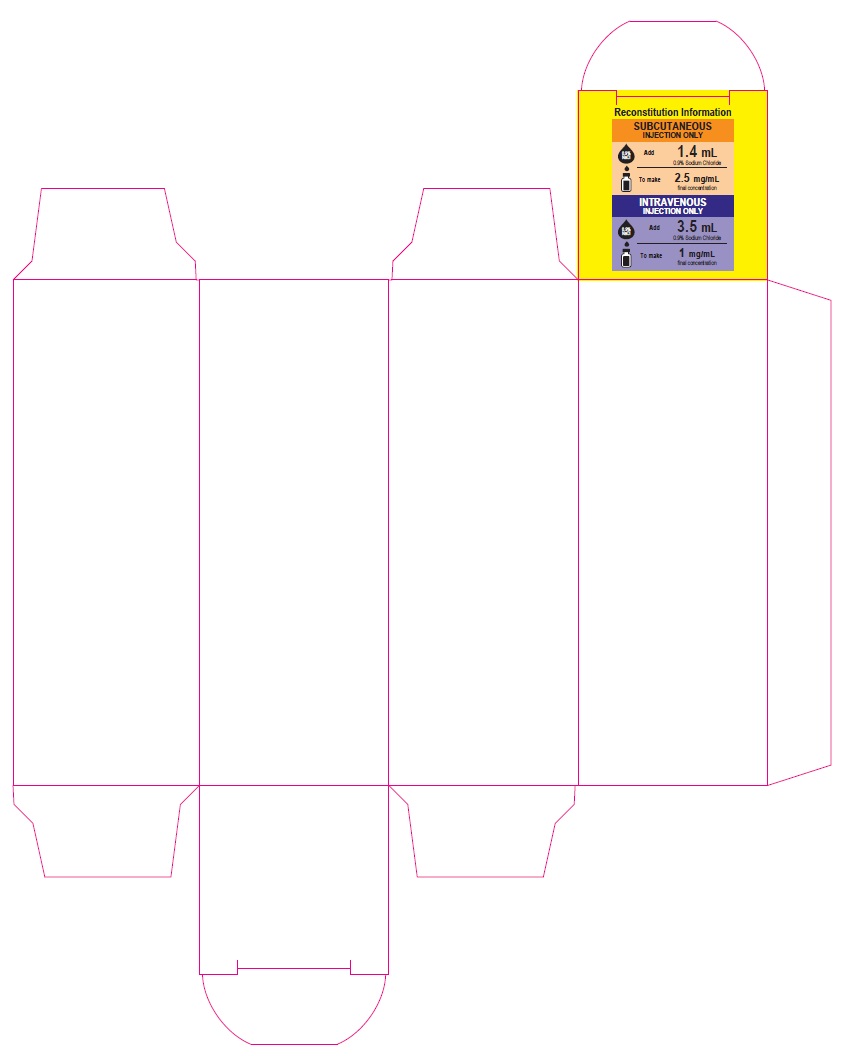
Reconstitution Information – Inside Flap (All cartons)
SUBCUTANEOUS INJECTION ONLY
0.9% NaCl
Add
1.4 mL
0.9% Sodium Chloride
To make
2.5 mg/mL
final concentration
INTRAVENOUS INJECTION ONLY
0.9% NaCl
Add
3.5 mL
0.9% Sodium Chloride
To make
1 mg/mL
final concentration