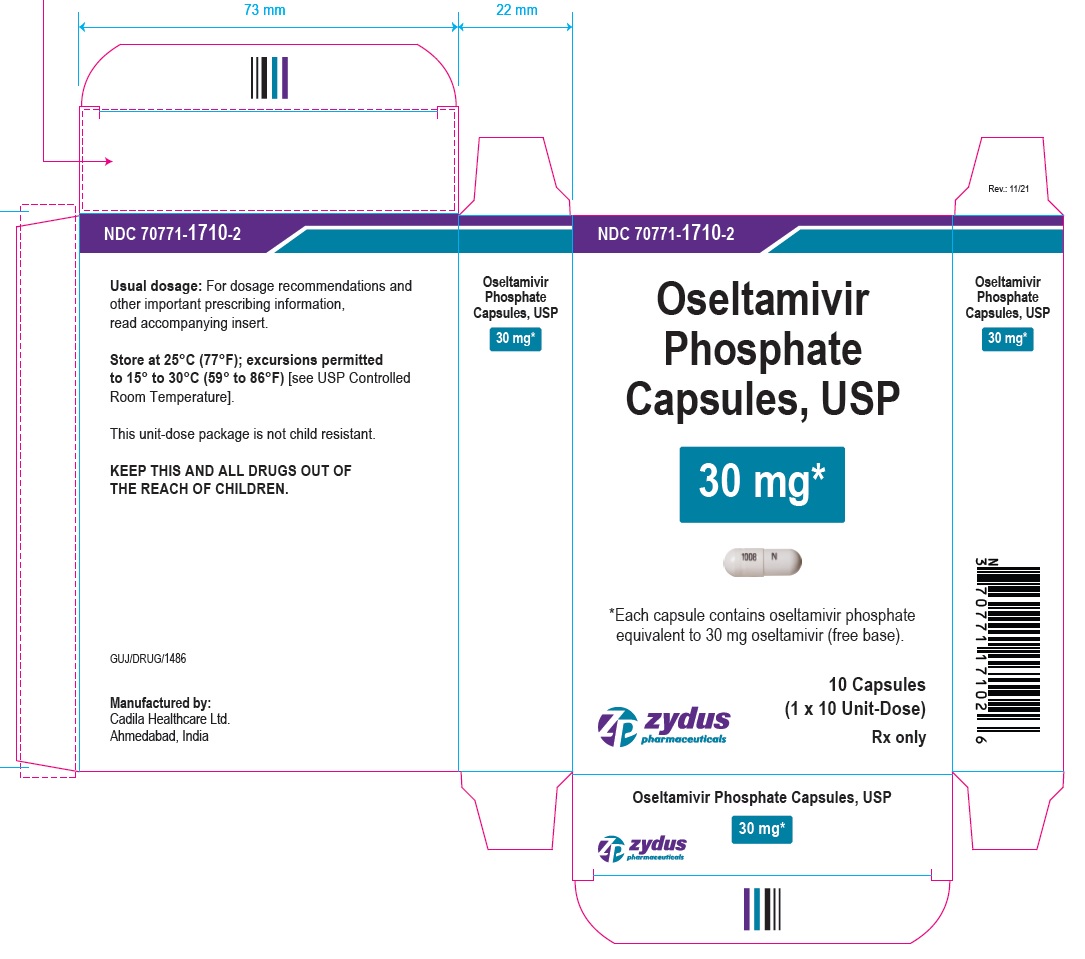
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
OSELTAMIVIR PHOSPHATE Capsules, USP
30* mg
*Each capsule contains oseltamivir phosphate equivalent to 30 mg oseltamivir (free base).
10 Capsules
(1 x 10 Unit-Dose)
Rx only
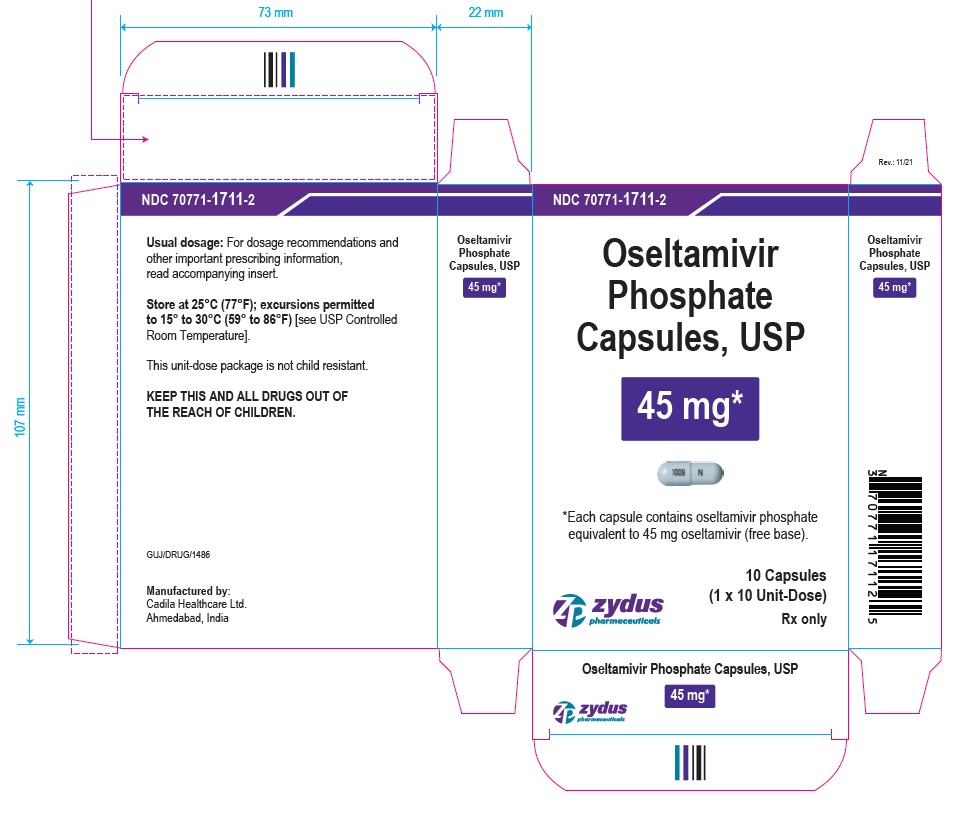
OSELTAMIVIR PHOSPHATE Capsules, USP
45* mg
*Each capsule contains oseltamivir phosphate equivalent to 45 mg oseltamivir (free base).
10 Capsules
(1 x 10 Unit-Dose)
Rx only
OSELTAMIVIR PHOSPHATE Capsules, USP
75* mg
*Each capsule contains oseltamivir phosphate equivalent to 75 mg oseltamivir (free base).
10 Capsules
(1 x 10 Unit-Dose)
Rx only
