DESCRIPTION
Each suppository for rectal administration contains 25 mg hydrocortisone acetate USP in a hydrogenated cocoglyceride base. Hydrocortisone acetate is a corticosteroid.
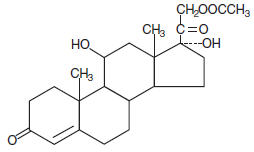
Chemically, hydrocortisone acetate is pregn-4-ene-3, 20 dione, 21- (acetyloxy)-11, 17-dihydroxy-, (11β)- with the following structural formula:
CLINICAL PHARMACOLOGY
In normal subjects, about 26 percent of hydrocortisone acetate is absorbed when the hydrocortisone acetate suppository is applied to the rectum. Absorption of hydrocortisone acetate may vary across abraded or inflamed surfaces.
Topical steroids are primarily effective because of their anti-inflammatory, antipruritic and vasoconstrictive action.
INDICATIONS AND USAGE
For use in inflamed hemorrhoids, post irradiation (factitial) proctitis, as an adjunct in the treatment of chronic ulcerative colitis, cryptitis, other inflammatory conditions of the anorectum, and pruritus ani.
CONTRAINDICATIONS
Hydrocortisone acetate suppositories are contraindicated in those patients with a history of hypersensitivity to any of the components.
PRECAUTIONS
Do not use unless adequate proctologic examination is made.
If irritation develops, the product should be discontinued and appropriate therapy instituted.
In the presence of an infection, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, the corticosteroid should be discontinued until the infection has been adequately controlled.
No long-term studies in animals have been performed to evaluate the carcinogenic potential of corticosteroid suppositories.
PREGNANCY CATEGORY C
In laboratory animals, topical steroids have been associated with an increase in the incidence of fetal abnormalities when gestating females have been exposed to rather low dosage levels. There are no adequate and well-controlled studies in pregnant women.
It is not known whether this drug is excreted in human milk.
Until adequate studies in pregnant or lactating women have been conducted, this drug should be used during pregnancy or by nursing mothers only when clearly needed and when the potential benefits outweigh the potential risks.
ADVERSE REACTIONS
The following local adverse reactions have been reported with corticosteroid suppositories:
|
|
DRUG ABUSE AND DEPENDENCE
Drug abuse and dependence have not been reported in patients treated with hydrocortisone acetate suppositories.