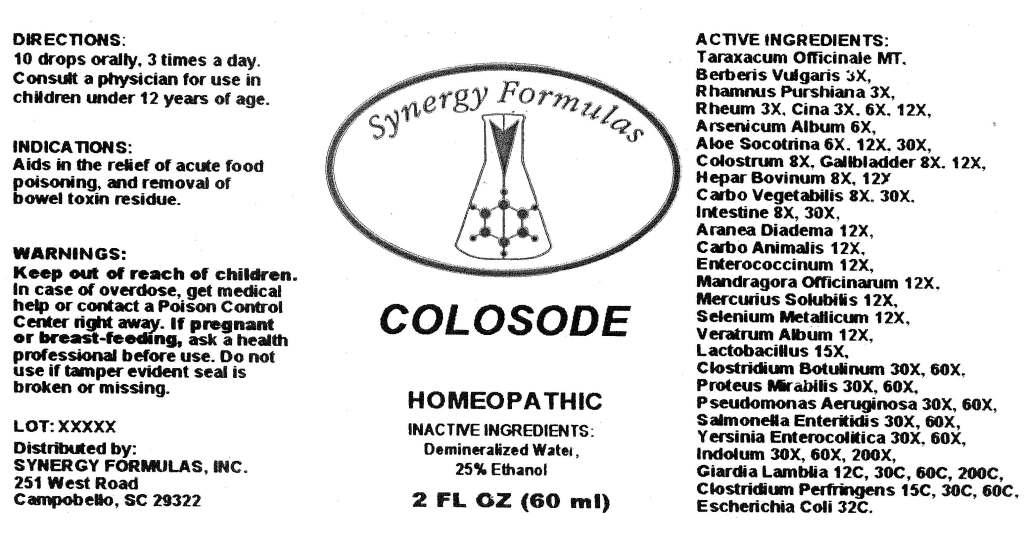
ACTIVE INGREDIENTS: Taraxacum officinale 1X, Berberis vulgaris 3X, Rhamnus purshiana 3X, Rheum 3X, Cina 3X, 6X, 12X, Arsenicum album 6X, Aloe socotrina 6X, 12X, 30X, Colostrum 8X, Gallbladder 8X, 12X, Hepar bovinum 8X, 12X, Carbo vegetabilis 8X, 30X, Intestine 8X, 30X, Aranea diadema 12X, Carbo animalis 12X, Enterococcinum 12X, Mandragora officinarum 12X, Mercurius solubilis 12X, Selenium metallicum 12X, Veratrum album 12X, Lactobacillus 15X, Clostridium botulinum 30X, 60X, Proteus mirabilis 30X, 60X, Salmonella enteritidis 30X, 60X, Pseudomonas aeruginosa 30X, 60X, Yersinia enterocolitica 30X, 60X, Indolum 30X, 60X, Clostridium perfringens 15C, 30C, 60C, Escherchia coli 32C, Giardia lamblia 12C, 30C, 60C, 200C.
WARNINGS: Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
If pregnant or breast-feeding, ask a health professional before use.
Do not use if tamper evident seal is broken or missing.
DIRECTIONS: 10 drops orally, 3 times a day. Consult a physician for use in children under 12 years of age.