PIZENSY- lactitol powder, for solution
Braintree Laboratories, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PIZENSY safely and effectively. See full prescribing information for PIZENSY.
PIZENSY (lactitol) for oral solution Initial U.S. Approval: 2020 INDICATIONS AND USAGEPIZENSY is an osmotic laxative indicated for the treatment of chronic idiopathic constipation (CIC) in adults. (1) DOSAGE AND ADMINISTRATIONDosage
Preparation and Administration
DOSAGE FORMS AND STRENGTHSADVERSE REACTIONSMost common adverse reactions (≥ 3%) are upper respiratory tract infection, flatulence, diarrhea, increased blood creatinine phosphokinase, abdominal distension, and increased blood pressure. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Braintree Laboratories, Inc. at 1-800-874-6756 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION. Revised: 8/2021 |
FULL PRESCRIBING INFORMATION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
- The recommended adult dosage of PIZENSY is 20 grams orally once daily, preferably with meals [see Clinical Pharmacology (12.3)].
- Reduce the dosage to 10 grams once daily for persistent loose stools.
- Administer oral medications at least 2 hours before or 2 hours after PIZENSY [see Drug Interactions (7.1)] .
2.2 Preparation and Administration Instructions
PIZENSY Multi-dose bottle
NOTE: The bottle top is a measuring cap marked to contain 10 grams of powder when filled to the top of white section in the cap marked by the arrow.
- Using the measuring cap, measure the prescribed dose.
- 20-gram dose: Fill the measuring cap twice to the top of the white section in cap marked by the arrow.
- 10-gram dose: Fill the measuring cap once to the top of the white section in cap marked by the arrow. - Pour the measured dose into an empty 8-ounce glass.
- Add 4 ounces to 8 ounces of water, juice or other common beverages (coffee, tea, soda) and stir to dissolve.
- Drink the entire contents of the glass.
PIZENSY Unit-dose packets
- Pour the contents of one or two unit-dose packets, as prescribed, into an empty 8-ounce glass.
- Add 4 ounces to 8 ounces of water, juice or other common beverages (coffee, tea, soda) to the glass containing the powder and stir thoroughly to dissolve.
- Drink the entire contents of the glass.
3. DOSAGE FORMS AND STRENGTHS
PIZENSY is a white to off-white crystalline powder for oral solution supplied as:
- 280 grams of lactitol in multi-dose bottles
- 560 grams of lactitol in multi-dose bottles
- 10 grams of lactitol in unit-dose packets
4. CONTRAINDICATIONS
PIZENSY is contraindicated in patients with:
- known or suspected mechanical gastrointestinal obstruction
- galactosemia
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to PIZENSY in 807 patients with CIC in a six-month placebo-controlled trial (Study 1), a three-month active-controlled trial (Study 2) [see Clinical Studies (14)] , and a one-year uncontrolled safety study (NCT02819310). Of the 298 patients in the one-year uncontrolled study, 55 patients were enrolled from Study 1 or Study 2.
Most Common Adverse Reactions:
Table 1 provides the incidence of adverse reactions in Study 1 reported in at least 3% of patients in the PIZENSY treatment group and at higher incidence than placebo.
|
1 reported in at least 3% of patients and greater than placebo |
||||
|
2 74 of 291 patients in the PIZENSY group at least temporarily reduced their dose |
||||
|
3 Upper respiratory tract infection includes the terms viral upper respiratory tract infection and nasopharyngitis. |
||||
|
4 Increased blood creatinine phosphokinase includes the term blood creatinine phosphokinase myocardial band (MB) increased. |
||||
|
5 Increased blood pressure includes the term Hypertension |
||||
|
PIZENSY 2 N=291 (%) |
Placebo N=302 (%) |
|||
| Upper Respiratory Tract Infection 3 | 9 | 6 | ||
| Flatulence | 8 | 3 | ||
| Diarrhea | 4 | 3 | ||
| Increased blood creatinine phosphokinase 4 | 4 | 3 | ||
| Abdominal Distension | 3 | 1 | ||
| Increased blood pressure 5 | 3 | 1 | ||
In Study 2, the safety profile of PIZENSY was similar to Study 1.
In the 1-year uncontrolled safety study, adverse reactions reported in patients receiving PIZENSY (N=298) with an incidence of at least 3% that are not represented in Table 1 include urinary tract infection (5%) and abdominal pain (3%).
Adverse Reaction of Special Interest - Severe Diarrhea
In Study 1, severe diarrhea was reported in 2 (1%) PIZENSY-treated patients compared to no patients in the placebo group.
Adverse Reactions Leading to Discontinuation
In Study 1, 11/291 (4%) PIZENSY-treated patients discontinued due to adverse reactions, compared to 10/302 (3%) of patients in the placebo group. The most common adverse reactions leading to discontinuation in PIZENSY-treated patients (1% each) were elevated creatinine kinase, flatulence, diarrhea and increased blood pressure.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of lactitol outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- hypersensitivity reactions, including rash and pruritus
7 DRUG INTERACTIONS
7.1 Reduced Absorption of Other Oral Medications
PIZENSY may reduce the absorption of concomitantly administered oral medications. Administer oral medications at least 2 hours before or 2 hours after PIZENSY [see Dosage and Administration (2.1)] .
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Lactitol is minimally absorbed systemically following oral administration [see Clinical Pharmacology (12.3)] , and it is unknown whether maternal use will result in fetal exposure to the drug. Available data from case reports on lactitol use in pregnant women are insufficient to evaluate for any drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal developmental studies, no effects on embryo-fetal development were observed with oral administration of lactitol to rats and rabbits during organogenesis at doses much higher than the maximum recommended human dosage.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Reproduction studies have been performed in pregnant rats at oral doses of lactitol up to 2 g/kg/day (about 0.93 times the recommended daily human dose based on body surface area) and in pregnant rabbits at oral doses up to 1 g/kg/day (about 0.93 times the recommended daily human dose based on body surface area) administered during the period of organogenesis. These studies did not reveal any evidence of harm to the fetus due to lactitol.
In a pre-and postnatal development study in rats, lactitol, administered from gestation day 6 to lactation day 20, did not cause any adverse effect on pre and postnatal development up to 2 g/kg/day (about 0.93 times the recommended daily human dose based on body surface area).
8.2 Lactation
Risk Summary
There are no data on the presence of lactitol in human or animal milk, the effects on the breastfed infant, or the effects on milk production. Lactitol is minimally absorbed systemically following oral administration [see Clinical Pharmacology (12.3)] . It is unknown whether the minimal systemic absorption of lactitol by adults will result in a clinically relevant exposure to breastfed infants. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for PIZENSY and any potential adverse effects on the breastfed infant from PIZENSY or from the underlying maternal condition.
11 DESCRIPTION
Lactitol is an osmotic laxative for oral use. Lactitol is a simple monosaccharide sugar alcohol. It is a dry, free flowing, white to off-white powder, readily soluble in aqueous solutions. As shown by the structure diagrams, it is an analog of the disaccharide lactulose. Its chemical name is 4-O-β-dGalactopyranosyl-d-glucitol lactitol.
Lactitol
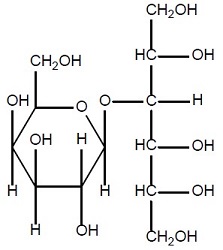
Molecular Formula C 12H 24O 11 Molecular Weight 344.31
PIZENSY (lactitol) for oral solution is available in unit-dose packets and multi-dose bottles. There are no inactive ingredients.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lactitol exerts an osmotic effect, causing the influx of water into the small intestine leading to a laxative effect in the colon.
12.3 Pharmacokinetics
Following a single oral dose of 20-gram PIZENSY in healthy adult subjects under fed conditions, the mean ± SD peak serum concentration (C max) is 776 ± 253 ng/mL, and the mean ± SD area under the curve (AUC) is 6,019 ± 1,771 ng*hr/mL.
Absorption
Lactitol is minimally absorbed systemically following oral administration. The mean ± SD time to reach peak serum concentration (T max) is 3.6 ± 1.2 hours following a single oral dose of 20-gram PIZENSY in healthy adult subjects under fed conditions.
Effect of Food
C max and AUC values increase greater than 2-fold under fasted conditions compared to fed conditions.
Elimination
The mean half-life of lactitol is 2.4 hours.
Excretion
Lactitol is minimally absorbed in the small intestine. Unabsorbed lactitol is expected to be degraded into organic acids in the colon and is minimally excreted in the feces.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of lactitol was assessed in rats and transgenic mice. In a 2-year carcinogenicity study in rats, lactitol was not tumorigenic at daily oral doses up to 2 g/kg/day (about 0.93 times the recommended daily human dose based on body surface area). In a 6-month carcinogenicity study in Tg.rasH2 mice at daily oral doses of lactitol up to 2 g/kg/day (about 0.47 times the recommended daily human dose based on body surface area), there were no drug-related neoplasms.
Published studies indicated that lactitol was negative in the Ames test, chromosome aberration test with cultured mammalian cells, and in vivo mouse micronucleus test.
Published studies indicated that lactitol did not cause any adverse effect on fertility and early embryonic development in rats at doses up to 10 g/kg/day (about 4.6 times the recommended daily human dose based on body surface area).
14 CLINICAL STUDIES
Study 1
PIZENSY was evaluated in a double-blind, randomized, placebo-controlled, multicenter clinical study in adult patients with symptoms of CIC (NCT02819297). PIZENSY (20 grams once daily) was compared to placebo in a 6-month treatment period. Patients who developed persistent diarrhea or loose stools were allowed to reduce their dosage of study drug to 10 grams once daily. Patients had a mean age of 52 years (range 18 to 88 years); 76% were female, 60% were white, and 31% were black.
To be eligible for the study, patients were required to meet modified Rome II criteria for at least 12 weeks (which need not be consecutive) in the preceding 12 months. Rome II criteria were modified for the study to allow enrollment of patients having less than 3 defecations per week, rarely having a loose stool without the use of laxatives, and not meeting criteria for IBS-C. In addition, patients were required to report at least one of the following symptoms:
- Straining during at least 25% of defecations
- Lumpy or hard stool in at least 25% of defecations
- Sensation of incomplete evacuations for at least 25% of defecations
Patients who met these criteria were also required to demonstrate the following during the 2-week screening period:
- Average less than 3 complete spontaneous bowel movements (CSBMs) over the 2-week screening period. A spontaneous bowel movement (SBM) was defined as a bowel movement which had occurred with no rescue laxative use in the prior 24 hours, and a CSBM was defined as a SMB that is associated with a sense of complete evaluation.
- No more than one SBM with a Bristol Stool Form Scale (BSFS) of 6
- No SBMs with a BSFS of 7
The efficacy of PIZENSY was assessed using a responder analysis and change-from-baseline in the CSBM endpoint. Efficacy was assessed using information provided by patients after each bowel movement using an electronic diary.
The primary efficacy analysis was based on the first 12 weeks of the 6-month treatment period for 594 patients. A responder was defined as a patient who had at least 3 CSBMs in a given week and an increase of at least 1 CSBM from baseline in the same week for at least 9 weeks out of the first 12-week treatment period and at least 3 of the last 4 weeks (Weeks 9-12). The responder rates are shown in Table 2.
|
CI = confidence interval |
||||
|
a 74 of 291 patients in the PIZENSY group at least temporarily reduced their dose |
||||
|
b primary endpoint defined as a patient who had at least 3 CSBMs in a given week and an increase of at least 1 CSBM from baseline in the same week for at least 9 weeks out of the 12-week treatment period and at least 3 of the last 4 weeks of the study |
||||
|
c p-value <0.05 for difference from control |
||||
|
PIZENSY N=291 a |
Placebo N=303 |
Treatment Difference (95% CI) |
||
| Responder b | 25% | 13% | 12% c (6.0, 18.5) | |
A responder analysis based on Weeks 13 to 24 of the treatment period (i.e., the proportion of responders for at least 9 weeks of the last 12 weeks and at least 3 of the last 4 weeks) showed results similar to the responder analysis of the first 12 weeks.
Improvements in the mean frequency of CSBMs/week were seen at Week 1 with improvement generally maintained through Week 12. The PIZENSY group had a mean increase of 0.8 CSBM/week from baseline to Week 12 over the placebo group.
Study 2
PIZENSY (20 grams once daily) was compared to an active control (lubiprostone 24 mcg twice daily) in a double-blind, double-dummy design study in adult patient with symptoms of CIC (NCT02481947). Patients had a mean age of 46 years (range 18 to 87 years); 80% were female, 66% were white, and 29% were black. The primary endpoint was the same as Study 1. The frequency of CSBMs/week for the PIZENSY group was consistent with results from Study 1.
Other Studies
Studies of varying design describing the efficacy of lactitol in increasing the frequency of bowel movements during short-term treatment of less than 4 weeks in patients with symptoms of CIC have been published.
16 HOW SUPPLIED/STORAGE AND HANDLING
PIZENSY is supplied in a white to off-white crystalline powder form, for oral administration following reconstitution. PIZENSY is available in three sizes:
- 280 grams of lactitol in multi-dose bottles (NDC 52268-600-01)
- 560 grams of lactitol in multi-dose bottles (NDC 52268-600-02)
- carton of 28 unit-dose packets each containing 10 grams of lactitol (NDC 52268-600-03)
The measuring cap on each multi-dose bottle is marked to contain 10 grams of powder when filled to the top of white section in cap and may be used to measure the appropriate PIZENSY dose.
Each bottle contains a white desiccant packet printed “Do Not Eat.”
Storage
Store at 20°C to 25°C (68° to 77°F). Excursions permitted between 15° to 30°C (59° to 86°F). See
USP controlled room temperature.
17 PATIENT COUNSELING INFORMATION
Persistent Loose Stools
Advise patients to stop PIZENSY and contact their healthcare provider if they experience persistent loose stools.
Distributed by Braintree Laboratories, Inc.
Braintree, MA 02185
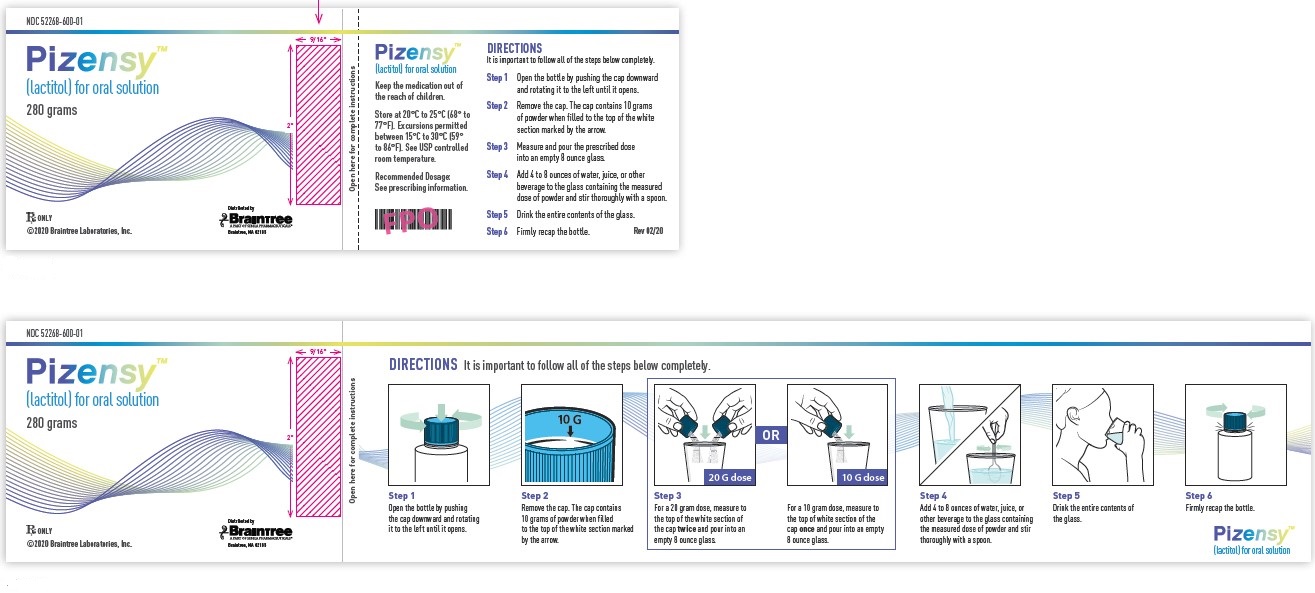
Principal Display Panel
NDC 52268-600-01
Pizensy™ (lactitol) for oral solution
280 grams
Rx ONLY
©2020 Braintree Laboratories, Inc.
Distributed by Braintree Laboratories
Keep the medication out of the reach of children.
Store at 20°C to 25°C (68° to 77°F). Excursions permitted between 15°C to 30°C (59° to 86°F). See USP controlled room temperature.
Recommended Dosage:
See prescribing information.
DIRECTIONS
It is important to follow all of the steps below completely.
Step 1: Open the bottle by pushing the cap downward and rotating it to the left until it opens.
Step 2: Remove the cap. The cap contains 10 grams of powder when filled to the top of the white section marked by the arrow.
Step 3: Measure and pour the prescribed dose into an empty 8 ounce glass.
Step 4: Add 4 to 8 ounces of water, juice, or other beverage to the glass containing the measured dose of powder and stir thoroughly with a spoon.
Step 5: Drink the entire contents of the glass.
Step 6: Firmly recap the bottle.
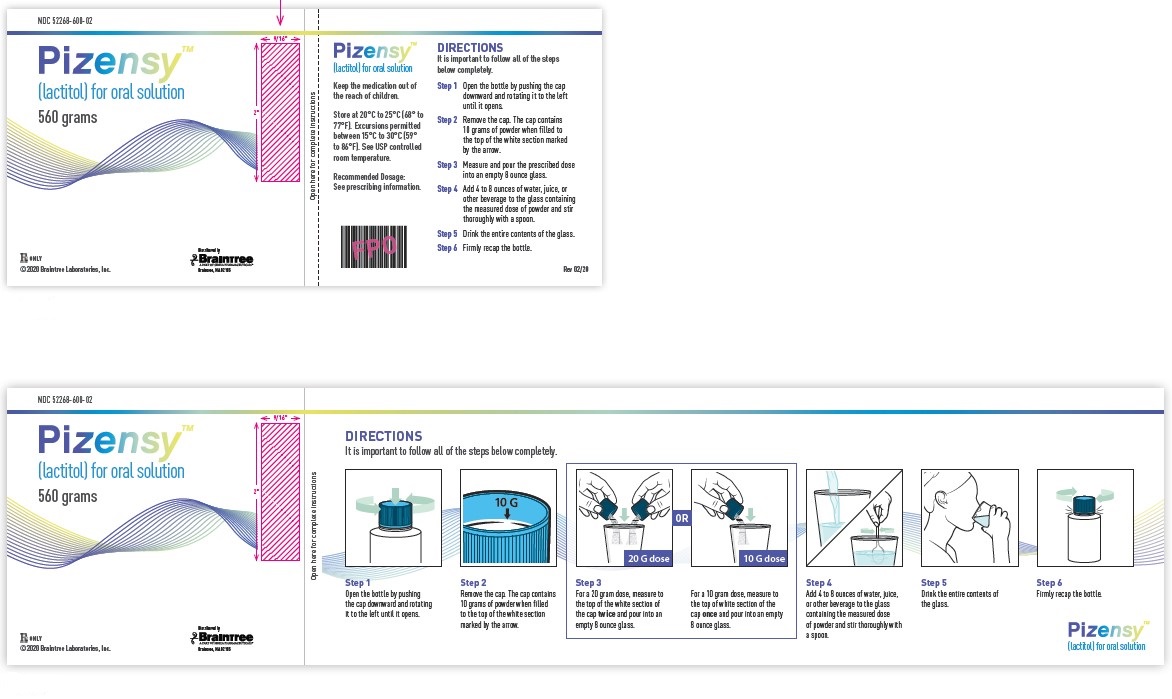
Principal Display Panel
NDC 52268-600-02
Pizensy™ (lactitol) for oral solution
560 grams
Rx ONLY
©2020 Braintree Laboratories, Inc.
Distributed by Braintree Laboratories
Keep the medication out of the reach of children.
Store at 20°C to 25°C (68° to 77°F). Excursions permitted between 15°C to 30°C (59° to 86°F). See USP controlled room temperature.
Recommended Dosage:
See prescribing information.
DIRECTIONS
It is important to follow all of the steps below completely.
Step 1: Open the bottle by pushing the cap downward and rotating it to the left until it opens.
Step 2: Remove the cap. The cap contains 10 grams of powder when filled to the top of the white section marked by the arrow.
Step 3: Measure and pour the prescribed dose into an empty 8 ounce glass.
Step 4: Add 4 to 8 ounces of water, juice, or other beverage to the glass containing the measured dose of powder and stir thoroughly with a spoon.
Step 5: Drink the entire contents of the glass.
Step 6: Firmly recap the bottle.
Principle Display Panel
NDC 52268-600-03
Pizensy™ (lactitol) for oral solution
10 grams per unit-dose packet
Rx ONLY
©2020 Braintree Laboratories, Inc.
Distributed by Braintree Laboratories
Keep the medication out of the reach of children.
Store at 20°C to 25°C (68° to 77°F). Excursions permitted between 15°C to 30°C (59° to 86°F). See USP controlled room temperature.
Recommended Dosage:
See prescribing information.
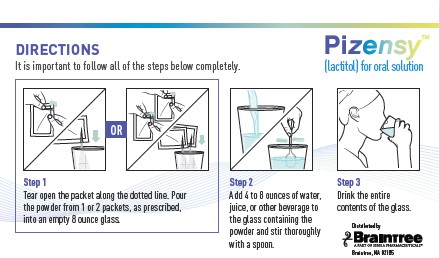
Directions
It is important to follow all of the steps below completely.
Step 1: Tear open the packet along the dotted line. Pour the powder from 1 or 2 packets, as prescribed, into an empty 8 ounce glass.
Step 2: Add 4 to 8 ounces of water, juice, or other beverage to the glass containing the powder and stir thoroughly with a spoon.
Step 3: Drink the entire contents of the glass.

Principle Display Panel
NDC 52268-600-04
Pizensy™ (lactitol) for oral solution
10 grams per unit-dose packet
Rx ONLY
©2020 Braintree Laboratories, Inc.
Distributed by Braintree Laboratories
Keep the medication out of the reach of children.
Store at 20°C to 25°C (68° to 77°F). Excursions permitted between 15°C to 30°C (59° to 86°F). See USP controlled room temperature.
This carton contains:
28 unit-dose packets
1 Patient Booklet
- Patient Instructions
- Full Prescribing Information
Recommended Dosage:
See prescribing information.
Directions
It is important to follow all of the steps below completely.
Step 1: Tear open the packet along the dotted line. Pour the powder from 1 or 2 packets, as prescribed, into an empty 8 ounce glass.
Step 2: Add 4 to 8 ounces of water, juice, or other beverage to the glass containing the powder and stir thoroughly with a spoon.
Step 3: Drink the entire contents of the glass.
| PIZENSY
lactitol powder, for solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Braintree Laboratories, Inc. (107904591) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Braintree Laboratories, Inc. | 617357954 | manufacture(52268-600) , analysis(52268-600) , label(52268-600) | |