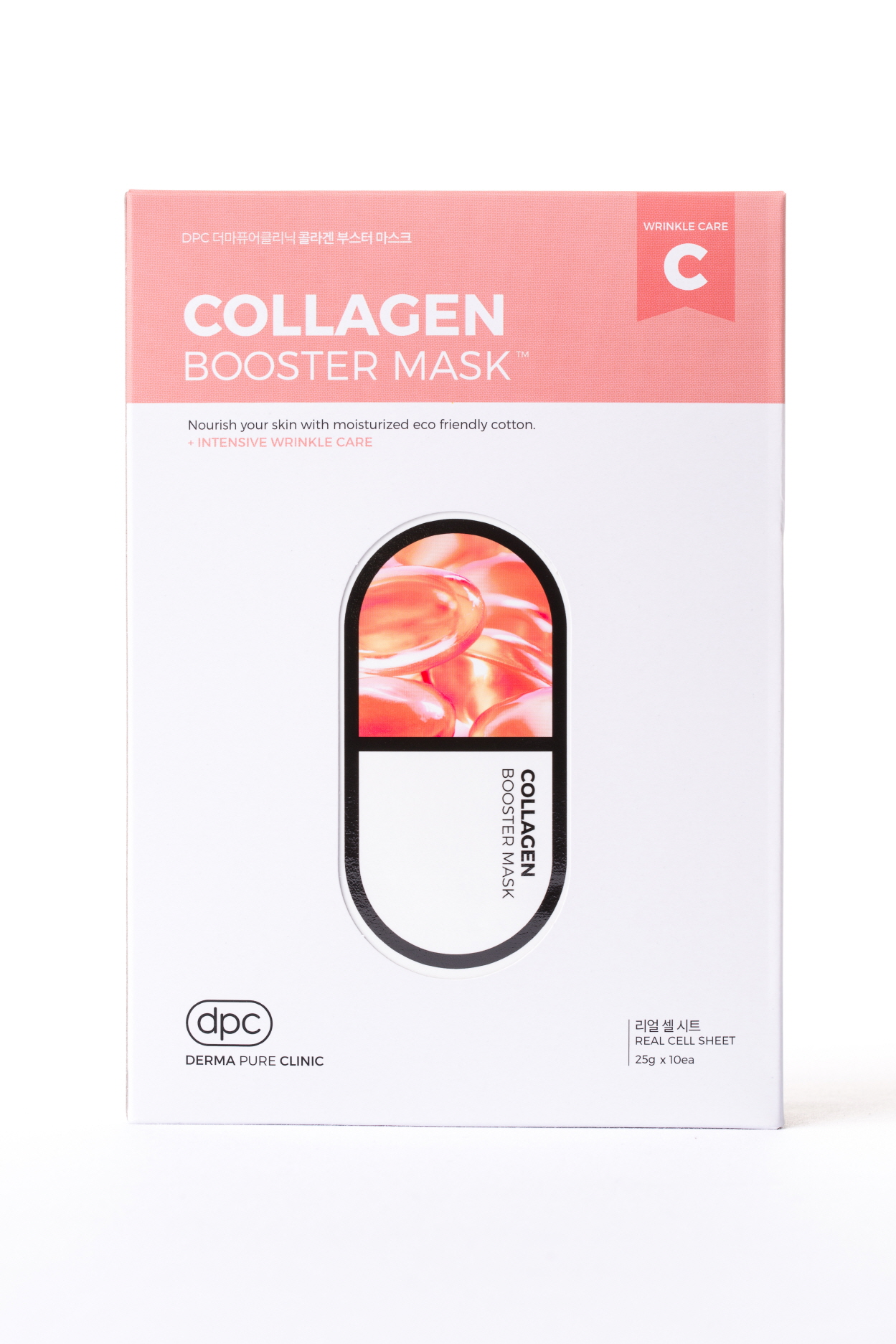
Water
Propanediol
Glycerin
Erythritol
Butylene Glycol
Caprylic/Capric Triglyceride
Dipotassium Glycyrrhizate
Disodium EDTA
Carbomer
Xanthan Gum
Hydroxyethylcellulose
Chlorphenesin
Sodium Hyaluronate
Squalane
Hydrolyzed Collagen
Simmondsia Chinensis (Jojoba) Seed Oil
Glycine Soja (Soybean) Seed Extract
Phaseolus radiatus seed extract
Thioctic Acid
Glutathione
Polyglutamic Acid
Methylpropanediol
Phenoxyethanol
Polyacrylate-13
Polyisobutene
Polysorbate 20
Sorbitan isostearate
Glyceryl Stearate
Polysorbate 60
Arginine
Citrus Grandis (Grapefruit) Seed Extract
Acorus Calamus Root Extract
Perilla Ocymoides Leaf Extract
1,2-Hexanediol
Caprylyl Glycol
Glycyrrhiza Glabra (Licorice) Root Extract
Zingiber Officinale (Ginger) Root Extract
Schizandra Chinensis Fruit Extract
Coptis Japonica Root Extract
Camellia Sinensis Leaf Extract
Fragrance
1. After cleansing wipe water and apply skin lotion on face.
2. Apply the mask evenly over the face for 10-20 minutes and remove.
3. Pad the left over residue lightly to absorb.