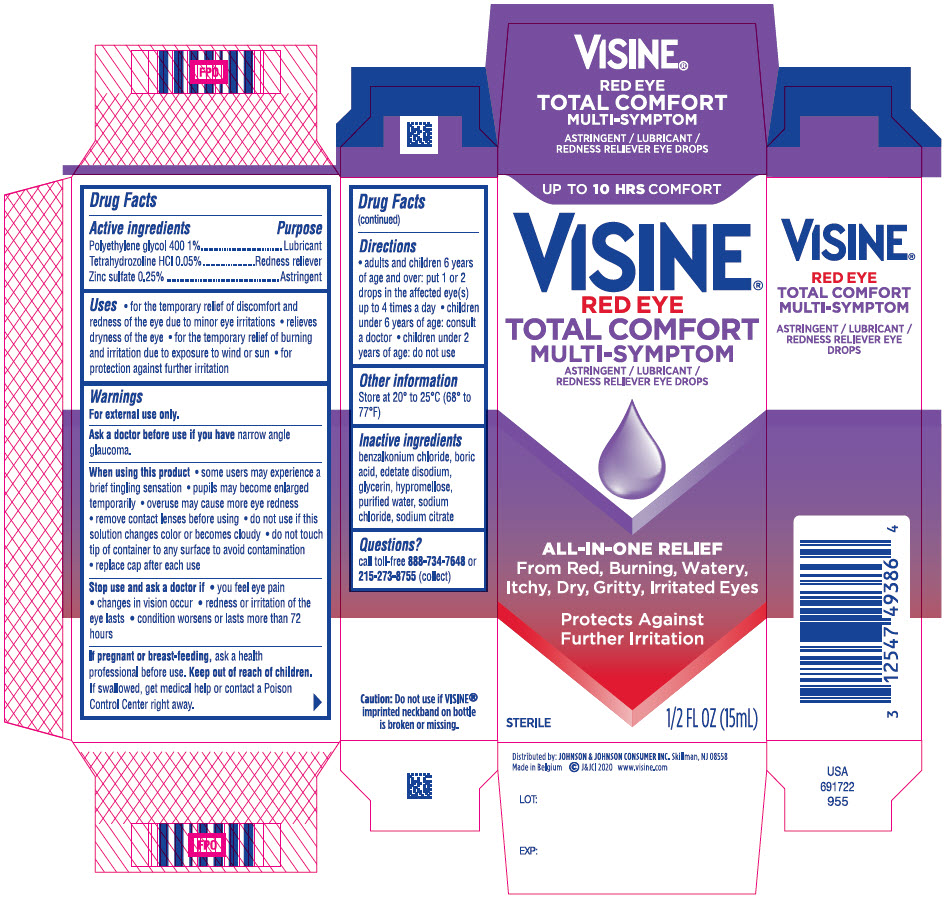
Uses
- for the temporary relief of discomfort and redness of the eye due to minor eye irritations
- relieves dryness of the eye
- for the temporary relief of burning and irritation due to exposure to wind or sun
- for protection against further irritation
Warnings
For external use only
When using this product
- some users may experience a brief tingling sensation
- pupils may become enlarged temporarily
- overuse may cause more eye redness
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Directions
- adults and children 6 years of age and over: put 1 or 2 drops in the affected eye(s) up to 4 times a day
- children under 6 years of age: consult a doctor
- children under 2 years of age: do not use
Inactive ingredients
benzalkonium chloride, boric acid, edetate disodium, glycerin, hypromellose, purified water, sodium chloride, sodium citrate
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
UP TO 10 HRS COMFORT
VISINE ®
RED EYE
TOTAL COMFORT
MULTI-SYMPTOM
ASTRINGENT /LUBRICANT
REDNESS RELIEVER EYE DROPS
ALL-IN ONE RELIEF
From Red, Burning, Watery,
Itchy, Dry, Gritty, Irritated Eyes
Protects Against
Further Irritation
STERILE
1/2 FL OZ (15 mL)