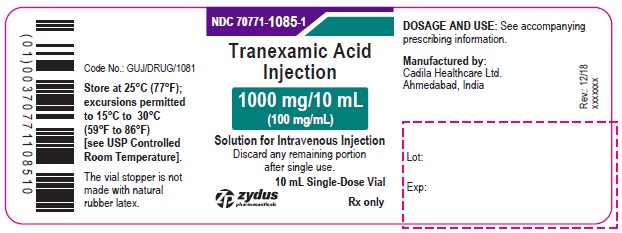
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1085-1
Tranexamic Acid Injection
1000 mg/10 mL
(100 mg/mL)
Solution for Intravenous Injection
Discard any remaining portion after single use.
10 mL Single-Dose Vial
Rx only
zydus pharmaceuticals
NDC 70771-1085-6
Tranexamic Acid Injection
1000 mg/10 mL
(100 mg/mL)
Solution for Intravenous Injection
Discard any remaining portion after single use.
10 X 10 mL Single-Dose Vials
Rx only
zydus pharmaceuticals
