5 DAY- aluminum chlorohydrate liquid
NUMARK BRANDS, INC
----------
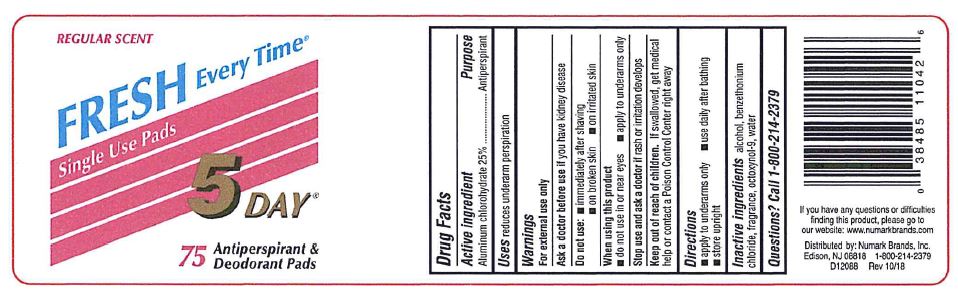
Drug Facts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
PRINCIPAL DISPLAY PANEL
REGULAR SCENT
FRESH EVERY TIME®
Single Use Pads
5 DAY®
75 Antiperspirant & Deodorant Pads
If you have any questions or difficulties
finding this product, please go to
our website: www.numarkbrands.com
Distributed by: Numark Brands, Inc.
Edison, NJ 08818 1-800-214-2379
D12088 Rev 10/18
| 5 DAY
aluminum chlorohydrate liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - NUMARK BRANDS, INC (080184668) |
Revised: 12/2023
Document Id: 3c76e18e-4dcd-4e23-9189-26e417178c7d
Set id: 571ca761-9696-7316-963d-c899897efa42
Version: 6
Effective Time: 20231218
NUMARK BRANDS, INC