280 GRAM Front Panel
Oxytet® Soluble
(oxytetracycline hydrochloride soluble powder)
Antibiotic
CAUTION: Federal (USA) law restricts this drug to use by or on
the order of a licensed veterinarian.
This packet contains 102.4 g of oxytetracycline hydrochloride and will make:
512 gallons (1936 liters) containing 200 mg oxytetracycline hydrochloride per gallon
128 gallons (484 liters) containing 800 mg oxytetracycline hydrochloride per gallon
This packet will treat 10,240 lbs of swine.
This packet will treat 4,096 lbs of turkey at 25 mg/lb body weight.
RECOMMENDED PACKET
STORAGE CONDITIONS:
Store between 20ºC - 25ºC
(68ºF - 77ºF) with excursions
permitted between 15ºC - 40ºC
(59ºF - 104ºF).
See back panel for instructions.
Net Weight: 9.87 oz (280 g)
For Use in Drinking Water Only
Not For Use in Liquid Medicated Feeds
Not For Use in Humans
Keep Out of Reach of Children
HAZARDOUS REMEDY
Restricted Drug (California) - Use
only as directed.
HUVEPHARMA®
Manufactured for:
Huvepharma, Inc.
525 Westpark Drive, Suite 230
Peachtree City, GA 30269
HUVEPHARMA and Oxytet
are registered trademarks of
Huvepharma EOOD
13925000 (Rev. 02-2021)
Approved by FDA under NADA #130-435
USE DIRECTIONS:
Mix fresh solutions daily. Use as the sole source of drinking water.
Do not mix this product directly with milk or milk replacers. Administer
1 hour before or 2 hours after feeding milk or milk replacers. As a
generalization, 200 chickens will drink 1 gallon of water per day for
each week of age. Turkeys will consume twice that amount. Administer up
to 5 days to swine and 7 to 14 days for chickens and turkeys. NOTE: The
concentration of drug required in medicated water must be adequate to
compensate for variation in the age of the animal, feed consumption rate,
and the environmental temperatures and humidity, each of which affects water
consumption.
CAUTION
Use as the sole source of oxytetracycline. Not to be used for more than 5 consecutive days in swine or 14
consecutive days in chickens and turkeys.
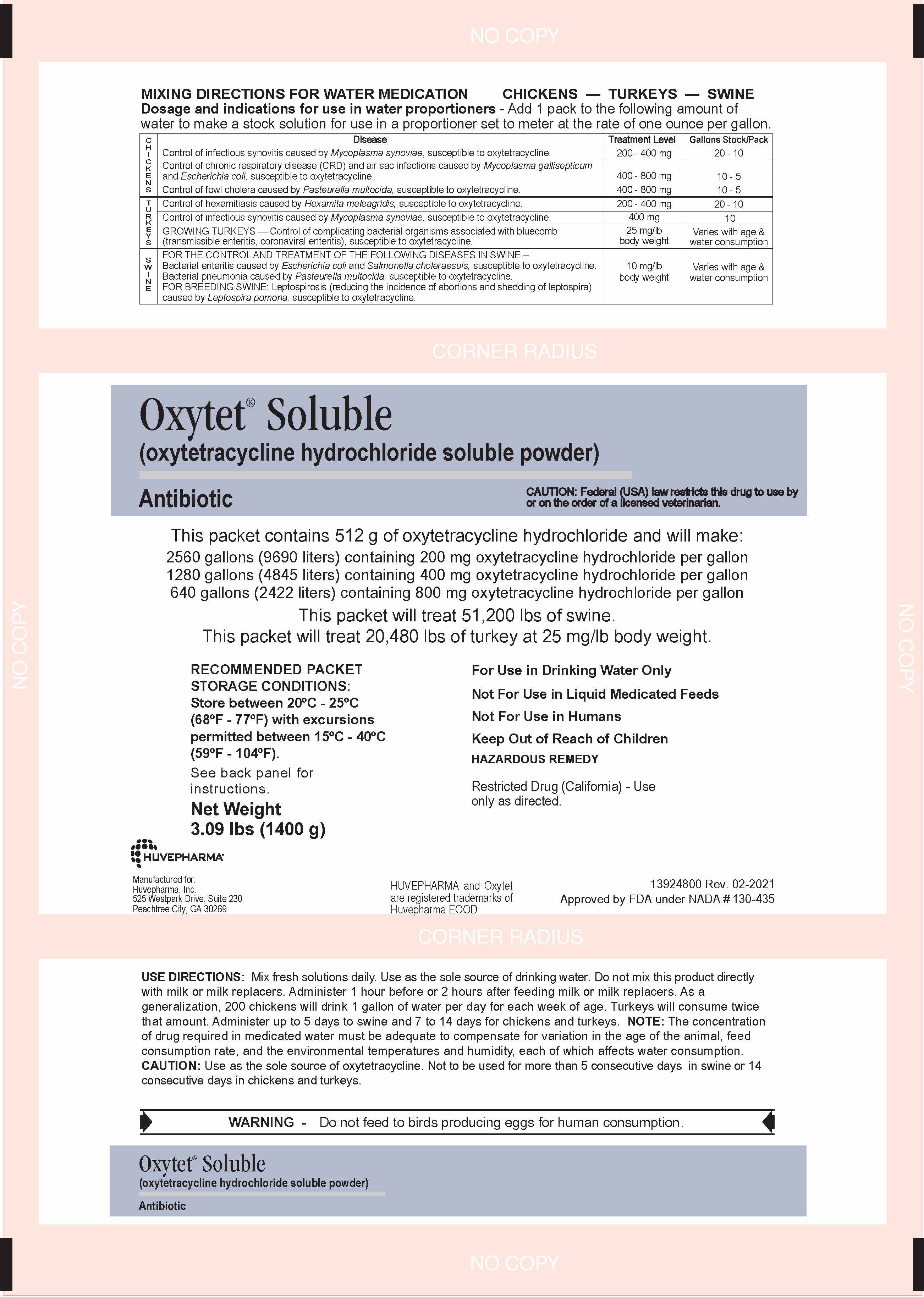
MIXING DIRECTIONS FOR WATER MEDICATION CHICKENS — TURKEYS — SWINE
Dosage and indications for use in water proportioners - Add the following amount to 1 gallon of stock solution when proportioner is set to meter at the rate of 1 oz per gallon.
|
C H I C K E N S | Disease | Treatment Level | Pack/Gallon Stock |
| Control of infectious synovitis caused by Mycoplasma synoviae, susceptible to oxytetracycline. | 200 - 400 mg | ¼ - ½ (70 - 140 g) | |
| Control of chronic respiratory disease (CRD) and air sac infections caused by Mycoplasma gallisepticum
and Escherichia coli, susceptible to oxytetracycline. | 400 - 800 mg | ½ - 1 (140 - 280 g) | |
| Control of fowl cholera caused by Pasteurella multocida, susceptible to oxytetracycline. | 400 - 800 mg | ½ - 1 (140 - 280 g) | |
|
T U R K E Y S | Control of hexamitiasis caused by Hexamita meleagridis, susceptible to oxytetracycline. | 200 - 400 mg | ¼ - ½ (70 - 140 g) |
| Control of infectious synovitis caused by Mycoplasma synoviae, susceptible to oxytetracycline. | 400 mg | ½ (140 g) | |
| GROWING TURKEYS — Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis), susceptible to oxytetracycline. |
25 mg/lb body weight |
Varies with age & consumption |
|
|
S W I N E | FOR THE CONTROL AND TREATMENT OF THE FOLLOWING DISEASES IN SWINE – Bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis, susceptible to oxytetracycline. Bacterial pneumonia caused by Pasteurella multocida, susceptible to oxytetracycline. FOR BREEDING SWINE: Leptospirosis (reducing the incidence of abortions and shedding of leptospira) caused by Leptospira pomona, susceptible to oxytetracycline. | 10 mg/lb body weight | Varies with age & water consumption |
1400 GRAM Front Panel
Oxytet® Soluble
(oxytetracycline hydrochloride soluble powder)
Antibiotic
CAUTION: Federal (USA) law restricts this drug to use by or on
the order of a licensed veterinarian.
This packet contains 512 g of oxytetracycline hydrochloride and will make:
2560 gallons (9690 liters) containing 200 mg oxytetracycline hydrochloride per gallon
1280 gallons (4845 liters) containing 400 mg oxytetracycline hydrochloride per gallon
640 gallons (2422 liters) containing 800 mg oxytetracycline hydrochloride per gallon
This packet will treat 51,200 lbs of swine.
This packet will treat 20,480 lbs of turkey at 25 mg/lb body weight.
RECOMMENDED PACKET
STORAGE CONDITIONS:
Store between 20ºC - 25ºC
(68ºF - 77ºF) with excursions
permitted between 15ºC - 40ºC
(59ºF - 104ºF).
See back panel for
instructions.
Net Weight
3.09 lbs (1400 g)
For Use in Drinking Water Only
Not For Use in Liquid Medicated Feeds
Not For Use in Humans
Keep Out of Reach of Children
HAZARDOUS REMEDY
Restricted Drug (California) - Use
only as directed.
HUVEPHARMA®
Manufactured for:
Huvepharma, Inc.
525 Westpark Drive, Suite 230
Peachtree City, GA 30269
HUVEPHARMA and Oxytet
are registered trademarks of
Huvepharma EOOD
13924800 Rev. 02-2021
Approved by FDA under NADA #130-435
USE DIRECTIONS:
Mix fresh solutions daily. Use as the sole source of drinking water.
Do not mix this product directly with milk or milk replacers. Administer
1 hour before or 2 hours after feeding milk or milk replacers. As a
generalization, 200 chickens will drink 1 gallon of water per day for
each week of age. Turkeys will consume twice that amount. Administer
up to 5 days to swine and 7 to 14 days for chickens and turkeys.
NOTE: The concentration of drug required in medicated water must be
adequate to compensate for variation in the age of the animal, feed
consumption rate, and the environmental temperatures and humidity,
each of which affects water consumption.
CAUTION:
Use as the sole source of oxytetracycline. Not to be used for more than 5 consecutive days in swine or 14
consecutive days in chickens and turkeys.
MIXING DIRECTIONS FOR WATER MEDICATION CHICKENS — TURKEYS — SWINE
Dosage and indications for use in water proportioners - Add 1 pack to the following amount of water to make a stock solution for use in a proportioner set to meter at the rate of one ounce per gallon.
| C
H I C K E N S | Disease | Treatment Level | Gallons Stock/Pack |
| Control of infectious synovitis caused by Mycoplasma synoviae, susceptible to oxytetracycline. | 200 - 400 mg | 20 - 10 | |
| Control of chronic respiratory disease (CRD) and air sac infections caused by Mycoplasma gallisepticum and Escherichia coli, susceptible to oxytetracycline. | 400 - 800 mg | 10 - 5 | |
| Control of fowl cholera caused by Pasteurella multocida, susceptible to oxytetracycline. | 400 - 800 mg | 10 - 5 | |
| T
U R K E Y S | Control of hexamitiasis caused by Hexamita meleagridis, susceptible to oxytetracycline. | 200 - 400 mg | 20 - 10 |
| Control of infectious synovitis caused by Mycoplasma synoviae, susceptible to oxytetracycline. | 400 mg | 10 | |
| GROWING TURKEYS — Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis), susceptible to oxytetracycline. | 25 mg/lb body weight |
Varies with age & water consumption |
|
| S
W I N E | FOR THE CONTROL AND TREATMENT OF THE FOLLOWING DISEASES IN SWINE – Bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis, susceptible to oxytetracycline. Bacterial pneumonia caused by Pasteurella multocida, susceptible to oxytetracycline. FOR BREEDING SWINE: Leptospirosis (reducing the incidence of abortions and shedding of leptospira) caused by Leptospira pomona, susceptible to oxytetracycline. | 10 mg/lb body weight | Varies with age & water consumption |
1772 GRAM Front Panel
Oxytet® Soluble
(oxytetracycline hydrochloride soluble powder)
Antibiotic
CAUTION: Federal (USA) law restricts this drug to use by
or on the order of a licensed veterinarian.
This packet contains 648 g of oxytetracycline hydrochloride and will make:
3240 gallons (12,265 liters) containing 200 mg oxytetracycline hydrochloride per gallon
1620 gallons (6,132 liters) containing 400 mg oxytetracycline hydrochloride per gallon
810 gallons (3,066 liters) containing 800 mg oxytetracycline hydrochloride per gallon
This packet will treat 64,800 lbs of swine.
This packet will treat 25,920 lbs of turkey at 25 mg/lb body weight.
RECOMMENDED PACKET
STORAGE CONDITIONS:
Store between 20ºC - 25ºC
(68ºF - 77ºF) with excursions
permitted between 15ºC - 40ºC
(59ºF - 104ºF).
See back panel for
instructions.
Net Weight
3.91 lbs (1772 g)
For Use in Drinking Water Only
Not For Use in Liquid Medicated Feeds
Not For Use in Humans
Keep Out of Reach of Children
HAZARDOUS REMEDY
Restricted Drug (California) - Use
only as directed.
HUVEPHARMA®
Manufactured for:
Huvepharma, Inc.
525 Westpark Drive, Suite 230
Peachtree City, GA 30269
HUVEPHARMA and Oxytet
are registered trademarks of
Huvepharma EOOD.
13925200 (Rev. 02-2021)
Approved by FDA under #130-435
USE DIRECTIONS:
Mix fresh solutions daily. Use as the sole source of drinking water.
Do not mix this product directly with milk or milk replacers. Administer 1 hour before
or 2 hours after feeding milk or milk replacers. As a generalization, 200 chickens
will drink 1 gallon of water per day for each week of age. Turkeys will consume twice
that amount. Administer up to 5 days to swine and 7 to 14 days for chickens and turkeys.
NOTE: The concentration of drug required in medicated water must be adequate to compensate
for variation in the age of the animal, feed consumption rate, and the environmental
temperatures and humidity, each of which affects water consumption.
CAUTION:
Use as the sole source of oxytetracycline. Not to be used for more than 5 consecutive days in swine or 14
consecutive days in chickens and turkeys.
MIXING DIRECTIONS FOR WATER MEDICATION CHICKENS — TURKEYS — SWINE
Dosage and indications for use in water proportioners - Add 1 pack to the following amount of water to make a stock solution for use in a proportioner set to meter at the rate of one ounce per gallon.
| C
H I C K E N S | Disease | Treatment Level | Gallons Stock/Pack |
| Control of infectious synovitis caused by Mycoplasma synoviae, susceptible to oxytetracycline. | 200 - 400 mg | 25.3 - 12.6 | |
| Control of chronic respiratory disease (CRD) and air sac infections caused by Mycoplasma gallisepticum and Escherichia coli, susceptible to oxytetracycline. | 400 - 800 mg | 12.6 - 6.3 | |
| Control of fowl cholera caused by Pasteurella multocida, susceptible to oxytetracycline. | 400 - 800 mg | 12.6 - 6.3 | |
| T
U R K E Y S | Control of hexamitiasis caused by Hexamita meleagridis, susceptible to oxytetracycline. | 200 - 400 mg | 25.3 - 12.6 |
| Control of infectious synovitis caused by Mycoplasma synoviae, susceptible to oxytetracycline. | 400 mg | 12.6 | |
| GROWING TURKEYS — Control of complicating bacterial organisms associated with bluecomb (transmissible enteritis, coronaviral enteritis), susceptible to oxytetracycline. | 25 mg/lb body weight | Varies with age & water consumption |
|
| S
W I N E | FOR THE CONTROL AND TREATMENT OF THE FOLLOWING DISEASES IN SWINE – Bacterial enteritis caused by Escherichia coli and Salmonella choleraesuis, susceptible to oxytetracycline. Bacterial pneumonia caused by Pasteurella multocida, susceptible to oxytetracycline. FOR BREEDING SWINE: Leptospirosis (reducing the incidence of abortions and shedding of leptospira) caused by Leptospira pomona, susceptible to oxytetracycline. | 10 mg/lb body weight | Varies with age & water consumption |