HEB READY TO USE LAXATIVE- sodium phosphate, dibasic, unspecified form and sodium phosphate, monobasic, unspecified form enema
HEB
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
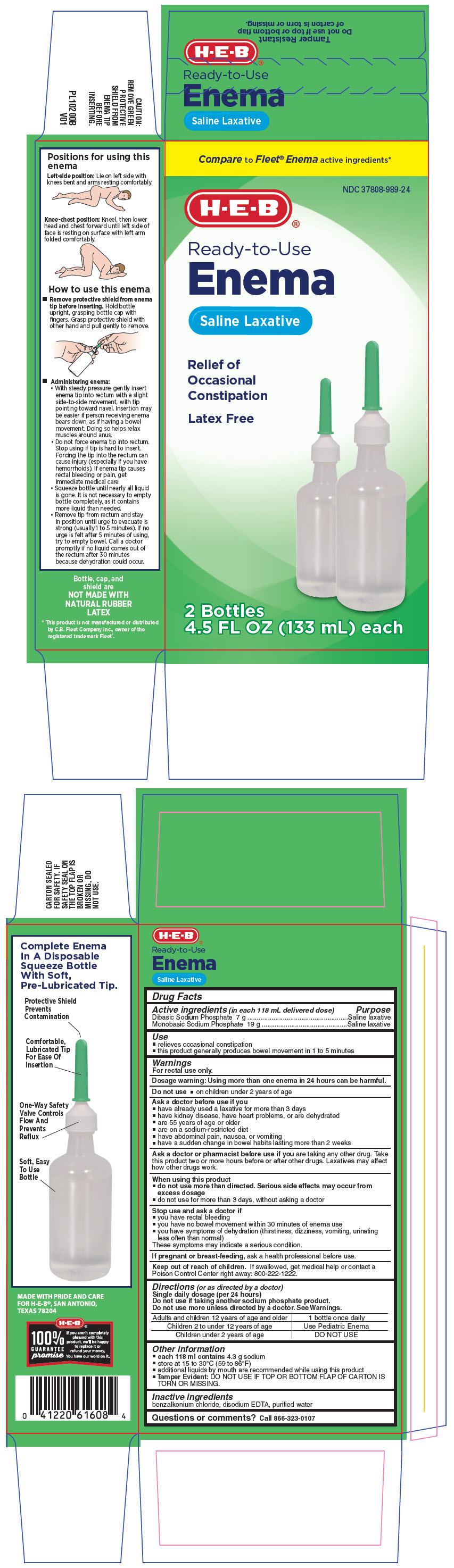
| Active ingredients (in each 118 mL delivered dose) | Purpose |
| Dibasic Sodium Phosphate 7 g | Saline laxative |
| Monobasic Sodium Phosphate 19 g | Saline laxative |
Use
- relieves occasional constipation
- this product generally produces bowel movement in 1 to 5 minutes
Warnings
For rectal use only.
Dosage warning
Using more than one enema in 24 hours can be harmful.
Do not use
- on children under 2 years of age
Ask a doctor before use if you
- have already used a laxative for more than 3 days
- have kidney disease, have heart problems, or are dehydrated
- are 55 years of age or older
- are on a sodium-restricted diet
- have abdominal pain, nausea, or vomiting
- have a sudden change in bowel habits lasting more than 2 weeks
Ask a doctor or pharmacist before use if you are taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
When using this product
-
do not use more than directed. Serious side effects may occur from excess dosage
- do not use for more than 3 days, without asking a doctor
Stop use and ask a doctor if
- you have rectal bleeding
- you have no bowel movement within 30 minutes of enema use
- you have symptoms of dehydration (thirstiness, dizziness, vomiting, urinating less often than normal)
These symptoms may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away: 800-222-1222.
Directions (or as directed by a doctor)
Single daily dosage (per 24 hours)
Do not use if taking another sodium phosphate product.
Do not use more unless directed by a doctor. See Warnings.
| Adults and children 12 years of age and older | 1 bottle once daily |
| Children 2 to under 12 years of age | Use Pediatric Enema |
| Children under 2 years of age | DO NOT USE |
Other information
-
each 118 ml contains 4.3 g sodium
- store at 15 to 30°C (59 to 86°F)
- additional liquids by mouth are recommended while using this product
-
Tamper Evident: DO NOT USE IF TOP OR BOTTOM FLAP OF CARTON IS TORN OR MISSING.
Inactive ingredients
benzalkonium chloride, disodium EDTA, purified water
Questions or comments?
Call 866-323-0107
PRINCIPAL DISPLAY PANEL - 2 Bottle Carton
Compare to Fleet® Enema active ingredients*
NDC 37808-989-24
H-E-B®
Ready-to-Use
Enema
Saline Laxative
Relief of
Occasional
Constipation
Latex Free
2 Bottles
4.5 FL OZ (133 mL) each
PRINCIPAL DISPLAY PANEL - 3 Bottle Carton
Compare to Fleet® Enema active ingredients*
NDC 37808-990-24
H-E-B®
Ready-to-Use
Enema
Saline Laxative
Relief of
Occasional
Constipation
Latex Free
3 Bottles
4.5 FL OZ (133 mL) each