VINIFERAMINE RENEWAL MOISTURIZER- dimethicone cream
VINIFERAMINE SILICONE BARRIER- dimethicone cream
VINIFERAMINE SKINMINERALZ- zinc oxide paste
VINIFERAMINE HYDROCORTISONE CREAM- hydrocortisone cream
VINIFERAMINE ANTIFUNGAL CREAM- miconazole nitrate cream
VINIFERAMINE CLEAN N MOIST- dimethicone lotion
VINIFERAMINE ANTISEPTIC CLEANSER- benzalkonium chloride spray
McCord Research
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
All products
See image within Principal Display Sections for each product
All products
See image within Principal Display Sections for each product
All products
See image within Principal Display Sections for each product
All products
See image within Principal Display Sections for each product
All products
See image within Principal Display Sections for each product
All products
See image within Principal Display Sections for each product
All products
See image within Principal Display Sections for each product
71358-011-01 (Sachet)
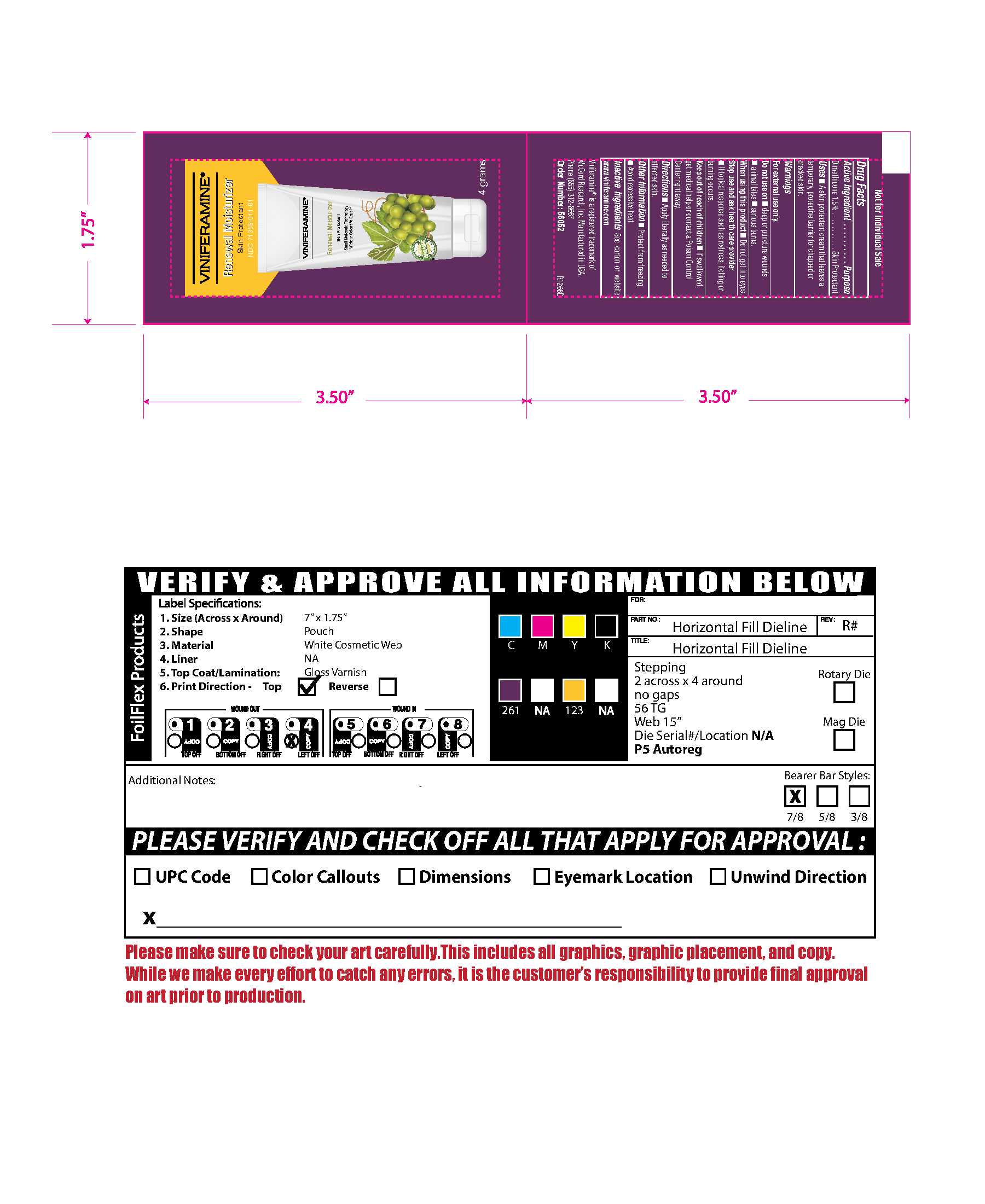
71358-011-01 (Box)

71358-011-02
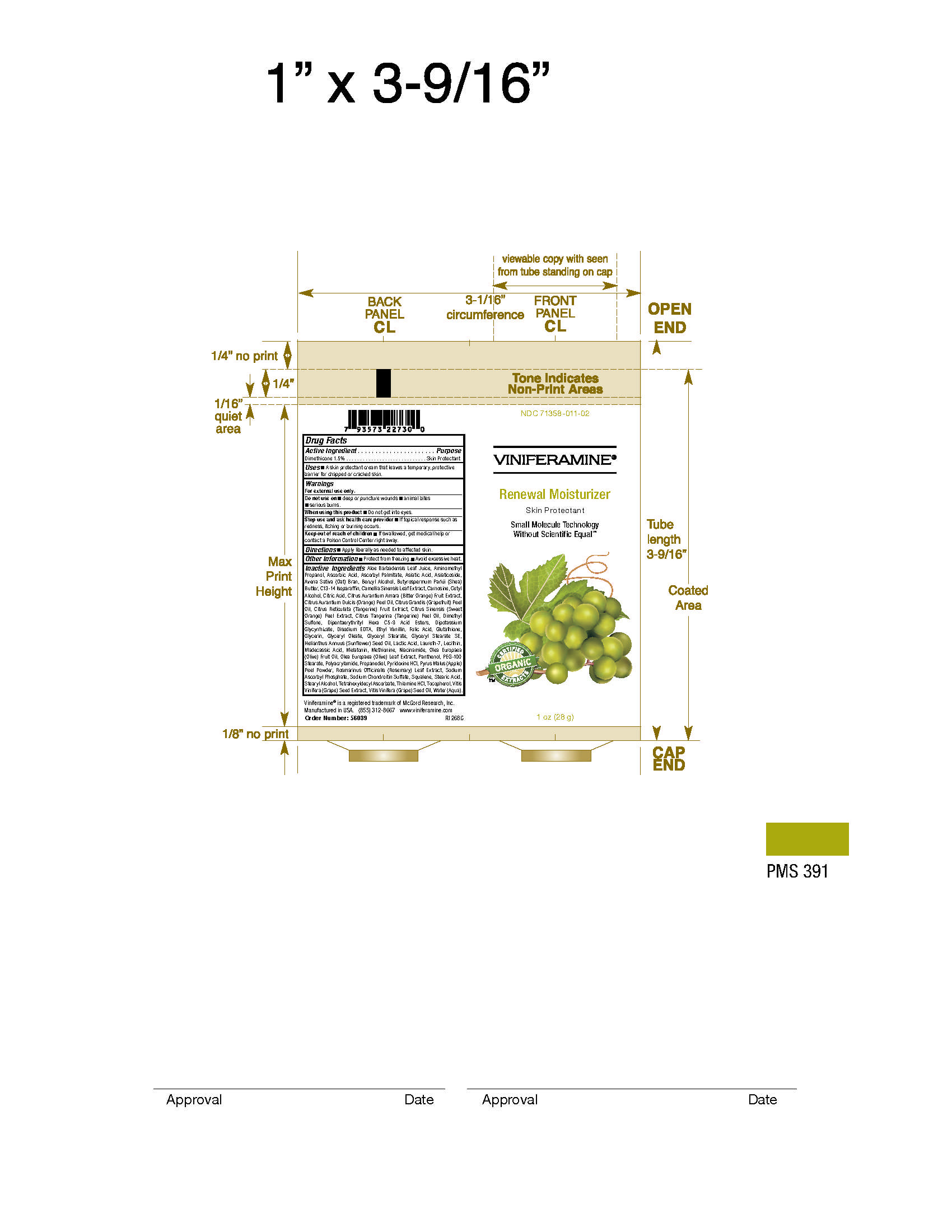
71358-011-03

71358-011-04

71358-066-01 (Sachet)
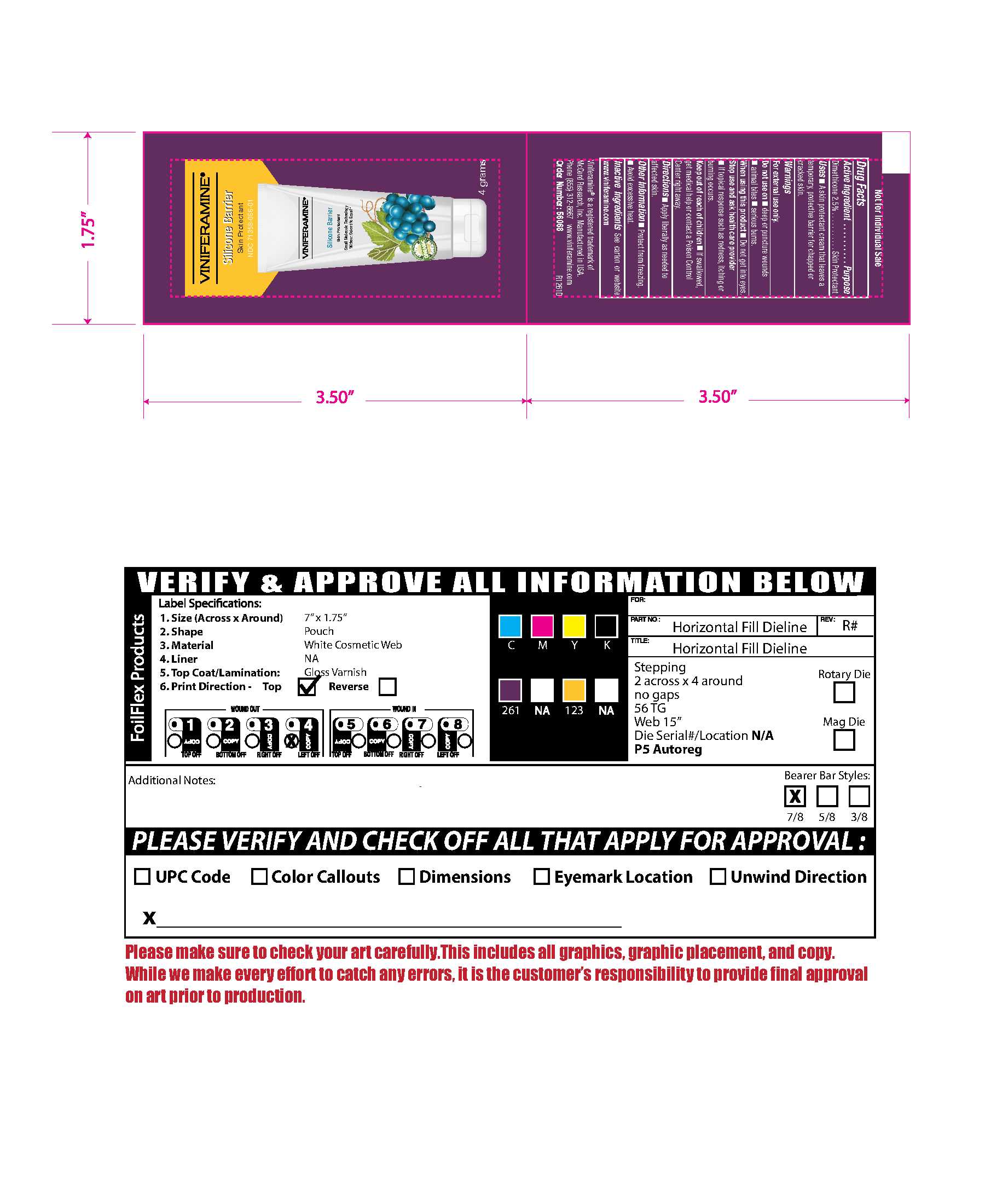
71358-066-01 (Box)

71358-066-02

71358-066-03

71358-012-01 (Sachet)

71358-012-01 (Box)

71358-012-02

71358-080-01

71358-077-01

71358-014-01

71358-015-01
