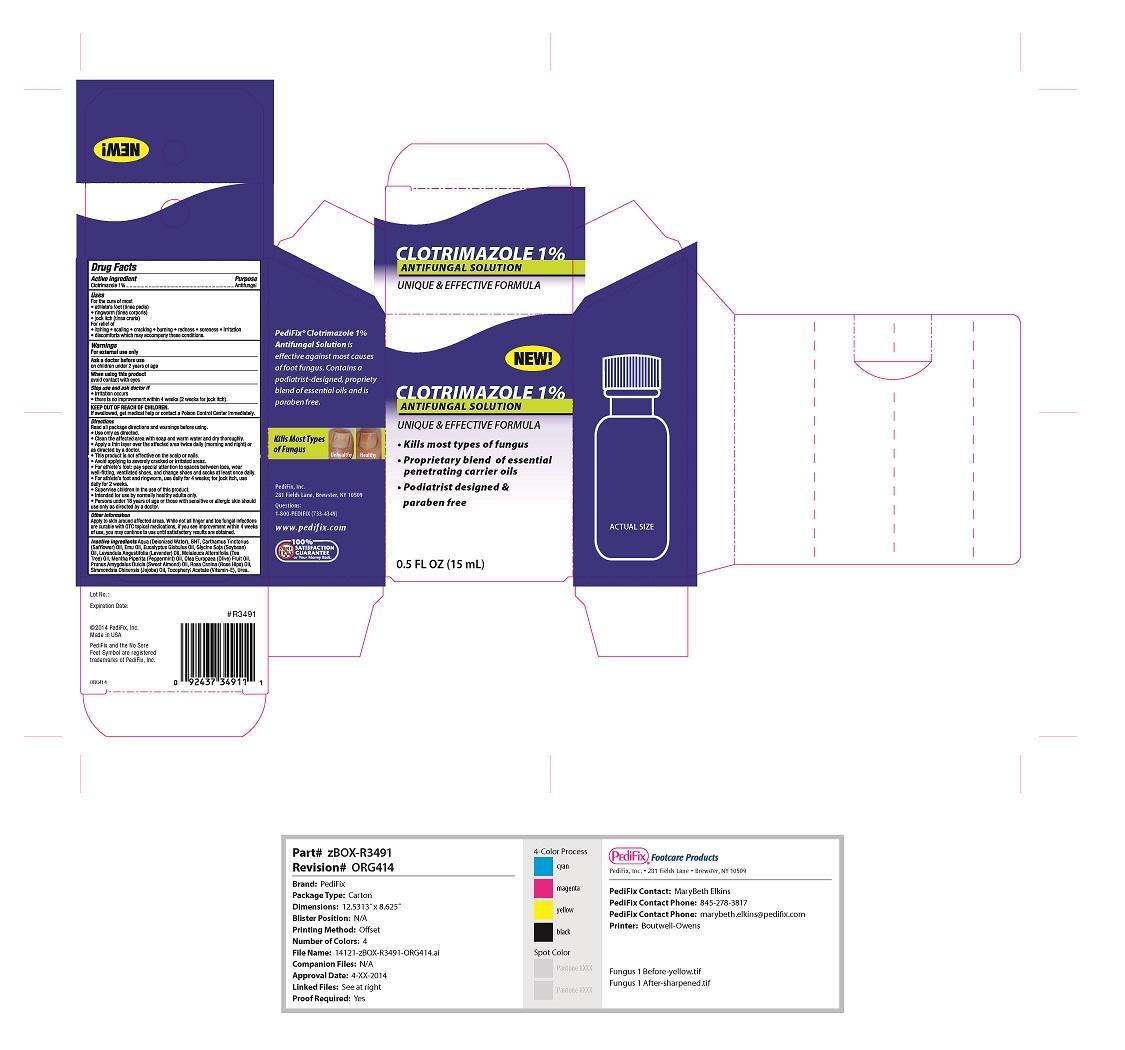
Warnings
For external use only
Ask a doctor before use on children under 2 years of age
When using this product avoid contact with eyes
Stop use and ask doctor if irritation occurs, there is no improvement within 4 weeks (2 weeks for jock itch)
If swallowed, get medical help or contact a poison control center immediately
Inactive ingredients
Aqua, BHT, safflower oil, emu oil, eucalyptus globlus oil, soybean oil, lavender oil, tea tree oil, peppermint oil, olive fruit oil, sweet almond oil, rose hip oil, jojoba oil, vitamin E, urea
Directions
Read all package directions and warnings before using:
Use only as directed
Clean the affected area with soap and warm water and dry throughly
Apply a thin layer over the affected area twice daily (morning and night) or as directed by a doctor
This product is not effective on the scalp or nails
Avoid applying to severly cracked or irritated areas
For athlete's foot pay special attention to spaces between toes, wear well-fitting vetilated shoes and change shoes and socks at least once daily
For athlete's foot and ringworm use daily for 4 weeks; for jock itch use daily for 2 weeks
Supervise children in the use of this product
Intended foruse by normally healthy adults only
Persons under 18 years of age or thoes with sensitive or allergic skin should use only as directed by a doctor.