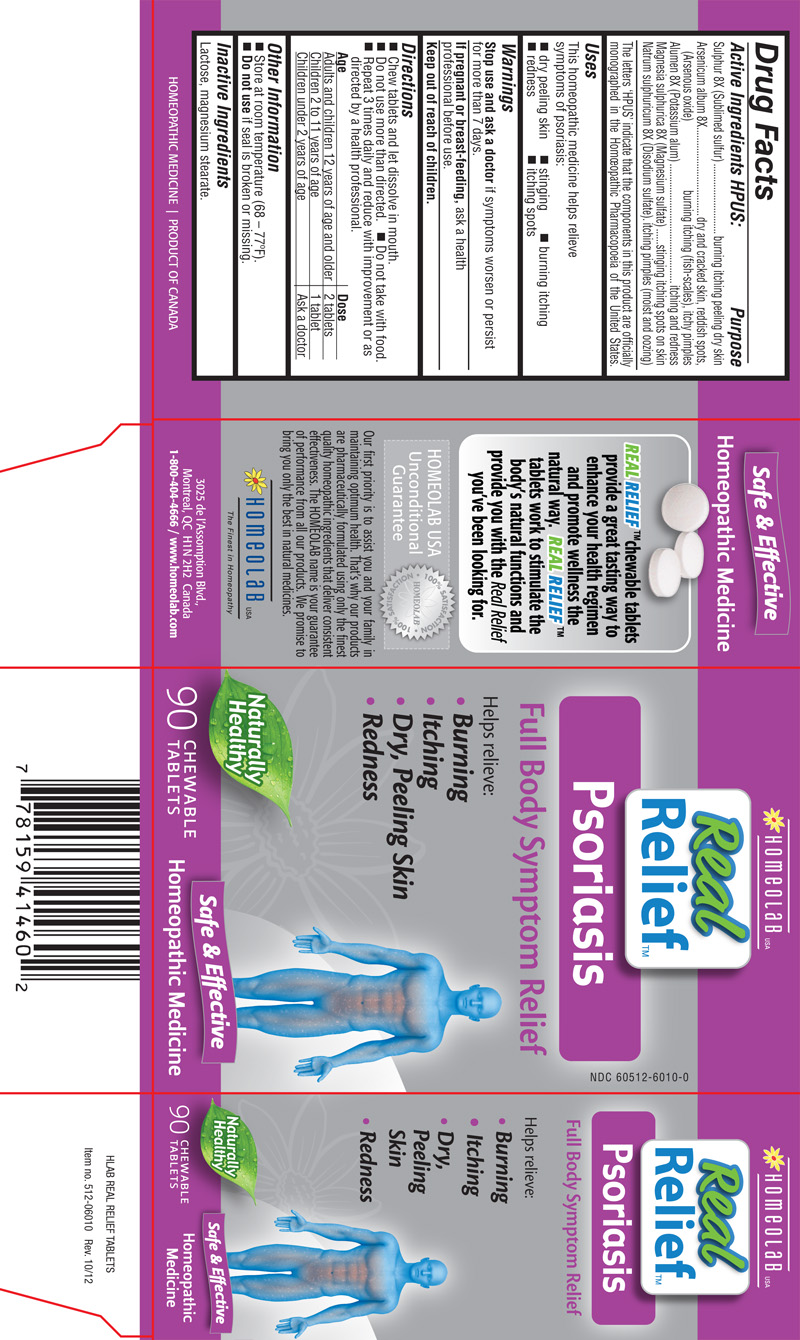
ACTIVE INGREDIENTS HPUS
Sulphur (Sublimed sulfur) 8XArsenicum album (Arsenous oxide) 8X
Alumen (Potassium alum) 8X
Magnesia sulphurica (Magnesium sulfate) 8X
Natrum sulphuricum (Disodium sulfate) 8X
The letters 'HPUS' indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
PURPOSE
burning itching peeling dry skindry and cracked skin, reddish spots, burning itching (fish-scales), itchy pimples
itching and redness
stinging itching spots on skin
itching pimples (moist and oozing)
USES
This homeopathic medicine helps relieve symptoms of psoriasis:
- dry peeling skin
- stinging
- burning itching
- redness
- itching spots
DIRECTIONS
Chew tablets and let dissolve in mouth.
Do not use more than directed.
Do not take with food.
Repeat 3 times daily and reduce with improvement or as directed by a health professional.
| Age | Dose |
|---|---|
| Adults and children 12 years of age and older | 2 tablets |
| Children 2 to 11 years of age | 1 tablet |
| Children under 2 years of age | Ask a doctor |