EFFECTIVE HAIR STRENGTHEN- glycerin solution
NUGGELA & SULE SL
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENTS PURPOSE
Glycerin 4.97%.................... Skin Protectant
Warnings
For external use only.
Do not use on damaged or broken skin.
Stop use and ask a doctor if rash occurs.
Keep out of reach of children.
Whhen using this product do not get into eyes.
Children under 6 months: as a doctor.
WARNINGS
Stop use and ask a doctor if rash occurs
Children under 6 months: ask a doctor
Warnings
Keep out of reach of children
Questions or comments?
+ 305 670 0979 M-F 9:00 am to 5:00 pm
Other Information
. keep the product in a cool and dry place
Directions
- follow the directions in the leaflet for proper use
- for better results, combine this product with Nuggela & Sule Premium shampoo and Conditioner

- follow the directions in the leaflet for proper use
- for better results, combine this product with Nuggela & Sule Premium shampoo and Conditioner
Uses
- regenerates and stimulates the hair
- nourish the scalp and provides the needed strenght to the broken and damaged hair
- provides skin protection to the scalp
Inactive Ingredients
AQUA, PROPYLENE GLYCOL, GLYCERIN, METHYLPROPANEDIOL, CAPRYLYL GLYCOL, GLYCOGEN, POLYQUATERNIUM-11, PEG-40 HYDROGENATED CASTOR OIL, DISODIUM EDTA , PARFUm, PHENOXYETHANOL, PHENYLPROPANOL, ALLIUM CEPA BULB EXTRACT, SODIUM SUCCINATE, POTASSIUM SORBATE, SODIUM BENZOATE, SODIUM HYDROXIDE, LINALOOL, CITRAL, HEXYL CINNAMAL, LIMONENE
Uses
- regenerates and stimulates the hair
- nourish the scalp and provides the needed strenght to the broken and damaged hair
- provides skin protection to the scalp
Package Label
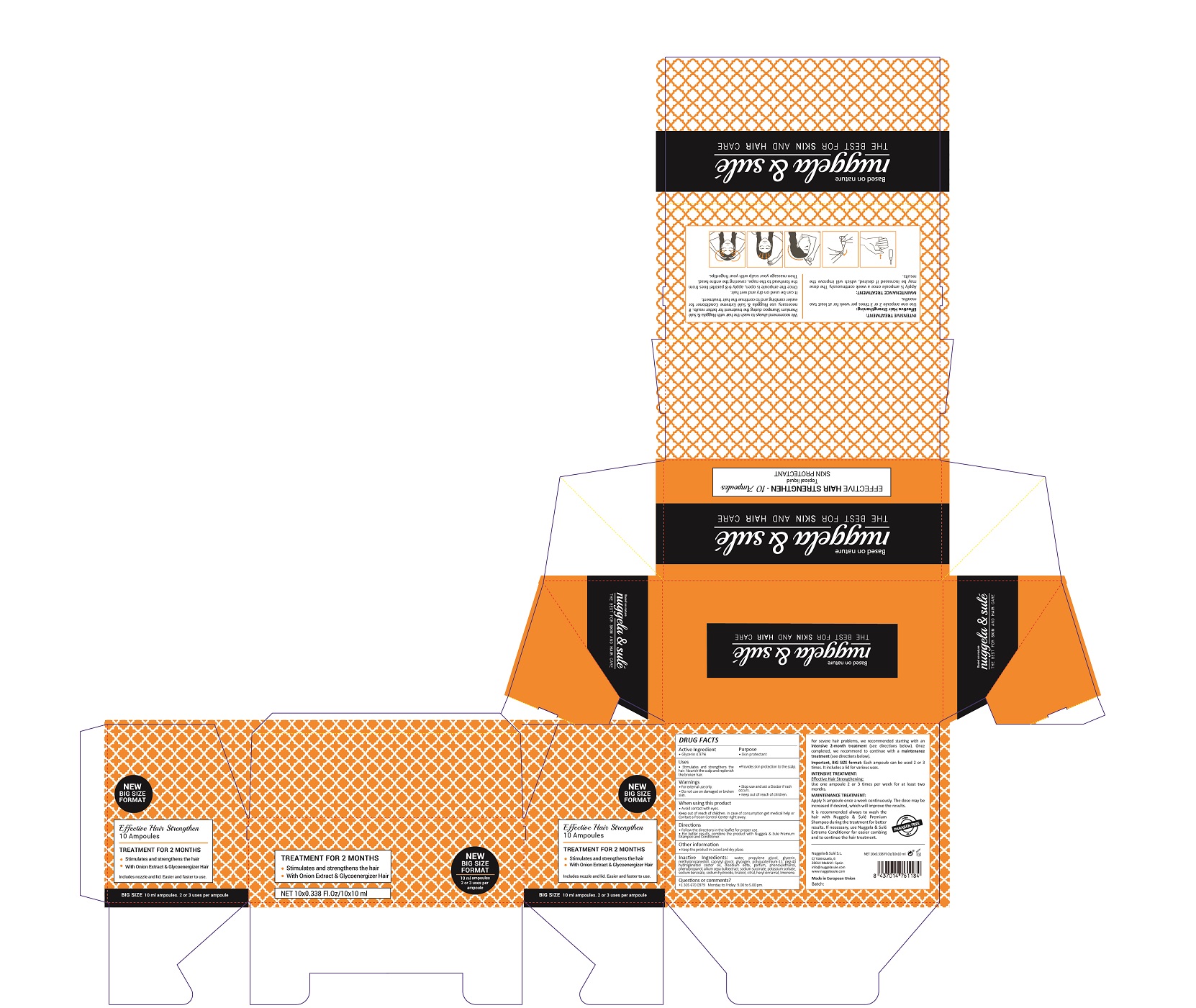
Treatment for two months
Stimulate and strenghthen the hair
With onion extract and Glycoenergizer hair

