FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Indication
CLOBEX Lotion, 0.05% is a super-high potent topical corticosteroid formulation indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses only in patients 18 years of age or older. Treatment should be limited to 2 consecutive weeks. For moderate to severe plaque psoriasis, treatment may be extended for an additional 2 weeks for localized lesions (less than 10% body surface area) that have not sufficiently improved after the initial 2-week treatment. Any additional benefits of extending treatment should be weighed against the risk of hypothalamic-pituitary-adrenal (HPA) axis suppression before prescribing for more than 2 weeks. The total dosage should not exceed 50 g (50 mL or 1.75 fl. oz.) per week.
Patients should be instructed to use CLOBEX Lotion, 0.05% for the minimum amount of time necessary to achieve the desired results [see Dosage and Administration (2)].
Use in patients under 18 years of age is not recommended due to numerically high rates of HPA axis suppression [see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)].
2 DOSAGE AND ADMINISTRATION
CLOBEX Lotion, 0.05% is for topical use only, and not for ophthalmic, oral or intravaginal use.
CLOBEX Lotion, 0.05% should be applied to the affected skin areas twice daily and rubbed in gently and completely.
The total dosage should not exceed 50 g (50 mL or 1.75 fl. oz.) per week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis.
CLOBEX Lotion, 0.05% contains a topical corticosteroid; therefore treatment should be limited to 2 consecutive weeks for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and up to 2 additional weeks in localized lesions (less than 10% body surface area) of moderate to severe plaque psoriasis that have not sufficiently improved after the initial 2 weeks of treatment with CLOBEX Lotion, 0.05%.
Unless directed by physician, CLOBEX Lotion, 0.05% should not be used with occlusive dressings.
3 DOSAGE FORMS AND STRENGTHS
Lotion, 0.05% w/w. Each gram of CLOBEX Lotion, 0.05%, contains 0.5 mg of clobestasol propionate in a white liquid.
5 WARNINGS AND PRECAUTIONS
5.1 Effect on the Endocrine System
Clobetasol propionate is a highly potent topical corticosteroid that has been shown to suppress the HPA axis at the lowest doses tested.
Systemic absorption of topical corticosteroids has caused reversible adrenal suppression with the potential for clinical glucocorticosteroid insufficiency after withdrawal of treatment. This may occur during treatment of upon withdrawal of the topical corticosteroid.
The effect of CLOBEX Lotion, 0.05% on HPA axis function was compared to clobetasol propionate cream 0.05% (Temovate E® Emollient, 0.05%) in adults in two trials, one for psoriasis and one for atopic dermatitis. In total, 8 of 10 evaluable subjects with moderate to severe plaque psoriasis experienced adrenal suppression following 4 weeks of CLOBEX Lotion, 0.05% therapy (treatment beyond 4 consecutive weeks is not recommended in moderate to severe plaque psoriasis.) In follow-up testing, 1 of 2 subjects remained suppressed after 8 days. In this comparative trial, for clobetasol propionate cream, 0.05% there were 3 of 10 evaluable subjects with HPA axis suppression.
Furthermore, 5 of 9 evaluable subjects with moderate to severe atopic dermatitis experienced adrenal suppression following 2 weeks of CLOBEX Lotion, 0.05% therapy (treatment beyond 2 consecutive weeks is not recommended in moderate to severe atopic dermatitis). Of the 3 subjects that had follow-up testing, one subject failed to recover adrenal function 7 days post-treatment. For subjects treated with clobetasol propionate cream, 0.05%, 4 of 9 evaluable subjects experienced adrenal suppression following 2 weeks of treatment. Of the 2 subjects that had follow-up testing, both recovered adrenal function 7 days post-treatment. The proportion of subjects suppressed may be underestimated because the adrenal glands were stimulated weekly with cosyntropin in these trials.
Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. Factors that predispose a patient using a topical corticosteroid to HPA axis suppression include the use of more potent steroids, use over large surface areas, use over prolonged periods, use under occlusion, use on an altered skin barrier, and use in patients with liver failure.
An adrenocorticotropic hormone (ACTH) stimulation test may be helpful in evaluating patients for HPA axis suppression. If HPA axis suppression is documented, an attempt should be made to gradually withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Manifestations of adrenal insufficiency may require supplemental systemic corticosteroids. Recovery of HPA axis function is generally prompt and complete upon discontinuation of topical corticosteroids.
Cushing’s syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids. Use of more than one corticosteroid-containing product at the same time may increase the total systemic exposure.
Pediatric patients may be more susceptible to systemic toxicity from use of topical corticosteroids. Use in patients under 18 years of age is not recommended due to numerically high rates of HPA axis suppression [see Use in Specific Populations (8.4)].
5.2 Local Adverse Reactions with Topical Corticosteroids
Local adverse reactions may occur more frequently with the use of occlusive dressings and higher potency corticosteroids, including clobetasol propionate. These reactions are listed in an approximate decreasing order of occurrence: folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, striae, miliaria, skin atrophy and telangiectasia. Some local adverse reactions may be irreversible. Clobetasol propionate is not recommended in patients with acne vulgaris, rosacea or perioral dermatitis.
5.3 Allergic Contact Dermatitis
Allergic contact dermatitis to any component of topical corticosteroids is usually diagnosed by a failure to heal rather than a clinical exacerbation. Clinical diagnosis of allergic contact dermatitis can be confirmed by patch testing.
5.4 Concomitant Skin Infections
In the presence of dermatologic infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, use of CLOBEX Lotion, 0.05%, should be discontinued until the infection has been adequately controlled.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled, clinical trials with CLOBEX Lotion, 0.05%, the following adverse reactions have been reported: burning/stinging, skin dryness, irritation, erythema, folliculitis, pruritus, skin atrophy, and telangiectasia. The pooled incidence of local adverse reactions in trials for psoriasis and atopic dermatitis with CLOBEX Lotion, 0.05% at 1% or greater was:
| Adverse Reaction | Incidence |
| Skin Atrophy | 4.2% |
| Telangiectasia | 3.2% |
| Discomfort Skin | 1.3% |
| Skin Dry | 1.0% |
Most local adverse events were rated as mild to moderate and they are not affected by age, race or gender.
Systemic absorption of topical corticosteroids has produced hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in some patients.
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been identified during post-approval use of CLOBEX Lotion, 0.05%.
- Endocrine disorders: Cushing’s syndrome, Adrenal suppression
- Skin: Rash, Pain of skin, Skin exfoliation, Skin chapped, Scaling, Induration/papulation, Lichenification.
- Other: Psoriasis (aggravation), Plaque Elevation, Excoriation.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C:
There are no adequate and well-controlled studies in pregnant women. Therefore, CLOBEX Lotion, 0.05% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application to laboratory animals.
Clobetasol propionate is absorbed percutaneously, and when administered subcutaneously it was a significant teratogen in both the rabbit and the mouse.
Clobetasol propionate has greater teratogenic potential than steroids that are less potent.
The effect of clobetasol propionate on pregnancy outcome and development of offspring was studied in the rat. Clobetasol propionate was administered subcutaneously to female rats twice daily (0, 12.5, 25, and 50 μg/kg/day) from day 7 of presumed gestation through day 25 of lactation or day 24 presumed gestation for those rats that did not deliver a litter. The maternal no-observed-effect level (NOEL) for clobetasol propionate was less than 12.5 μg/kg/day due to reduced body weight gain and feed consumption during the gestation period. The reproductive NOEL in the dams was 25 μg/kg/day (ratio of animal dose to proposed human dose of 0.07 on a mg/m2/day basis) based on prolonged delivery at a higher dose level. The no-observed-adverse-effect-level (NOAEL) for viability and growth in the offspring was 12.5 μg/kg/day (ratio of animal dose to proposed human dose of 0.03 on a mg/m2/day basis) based on incidence of stillbirths, reductions in pup body weights on days 1 and 7 of lactation, increased pup mortality, increases in the incidence of umbilical hernia, and increases in the incidence of pups with cysts on the kidney at higher dose levels during the preweaning period. The weights of the epididymides and testes were significantly reduced at higher dosages. Despite these changes, there were no effects on the mating and fertility of the offspring.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Because many drugs are excreted in human milk, caution should be exercised when CLOBEX Lotion, 0.05% is administered to a nursing woman.
8.4 Pediatric Use
Use of CLOBEX Lotion, 0.05% in pediatric patients is not recommended due to the potential for HPA axis suppression [see Warnings and Precautions (5.1)].
The HPA axis suppression potential of CLOBEX Lotion, 0.05% has been studied in adolescents (12 to 17 years of age) with moderate to severe atopic dermatitis covering a minimum of 20% of the total body surface area. In total 14 subjects were evaluated for HPA axis function. Subjects were treated twice daily for 2 weeks with CLOBEX Lotion, 0.05%. After 2 weeks of treatment, 9 out of 14 of the subjects experienced adrenal suppression. One out of 4 subjects treated with CLOBEX Lotion, 0.05% who were retested remained suppressed two weeks post-treatment. In comparison, 2 of 10 subjects treated with clobetasol propionate cream, 0.05% demonstrated HPA axis suppression. One subject who was retested recovered.
None of the subjects who developed HPA axis suppression had concomitant clinical signs of adrenal suppression and none of them was discontinued from the study for reasons related to the safety or tolerability of CLOBEX Lotion, 0.05%. However patients with acute illness or injury may have increased morbidity and mortality with intermittent HPA axis suppression.
Because of higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of glucocorticosteroid insufficiency during and/or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children.
HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
8.5 Geriatric Use
Clinical studies of CLOBEX Lotion, 0.05% did not include sufficient numbers of subjects aged 65 and over to adequately determine whether they respond differently than younger subjects. In general, dose selection for an elderly patient should be made with caution, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
Topically applied CLOBEX Lotion, 0.05% can be absorbed in sufficient amount to produce systemic effects [see Warnings and Precautions (5.1)].
11 DESCRIPTION
CLOBEX (clobetasol propionate) Lotion, 0.05% contains clobetasol propionate, a synthetic fluorinated corticosteroid, for topical use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and antipruritic agents. Clobetasol propionate is 21-chloro-9-fluoro-11β, 17-dihydroxy-16β -methylpregna-1,4-diene-3,20-dione 17-propionate, with the empirical formula C25H32CIFO5, and a molecular weight of 466.98 (CAS Registry Number 25122-46-7).
The following is the chemical structure:
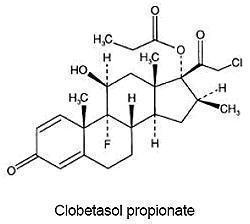
Clobetasol propionate is a white to almost white crystalline powder that is practically insoluble in water. Each gram of CLOBEX Lotion, 0.05% contains 0.5 mg of clobetasol propionate, in a white liquid composed of hypromellose, propylene glycol, mineral oil, polyoxyethylene glycol 300 isostearate, carbomer 1342, sodium hydroxide and purified water.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Like other topical corticosteroids CLOBEX Lotion, 0.05% has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids in general is unclear. However, corticosteroids are thought to act by induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
12.2 Pharmacodynamics
Vasoconstrictor Assay
CLOBEX Lotion, 0.05% is in the super-high range of potency as demonstrated in vasoconstrictor studies in healthy subjects when compared with other topical corticosteroids. However, similar blanching scores do not necessarily imply therapeutic equivalence.
Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression
In studies evaluating the potential for hypothalamic-pituitary-adrenal (HPA) axis suppression, CLOBEX Lotion, 0.05% demonstrated rates of suppression that were numerically higher than those of a clobetasol propionate 0.05% cream (Temovate E® Emollient, 0.05%), [see Warnings and Precautions (5.1) and Use in Specific Population (8.4)].
12.3 Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle, the integrity of the epidermal barrier and occlusion.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and other disease processes in the skin may increase percutaneous absorption.
There are no human data regarding the distribution of corticosteroids to body organs following topical application. Nevertheless, once absorbed through the skin, topical corticosteroids are handled through metabolic pathways similar to systemically administered corticosteroids. They are metabolized, primarily in the liver, and are then excreted by the kidneys. In addition, some corticosteroids and their metabolites are also excreted in the bile.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Clobetasol propionate was not carcinogenic to rats when topically applied for 2 years at concentrations up to 0.005% which corresponded to doses up to 11 µg/kg/day (ratio of animal dose to proposed human dose of 0.03 on a mg/m2/day basis).
Clobetasol propionate at concentrations up to 0.001% did not increase the rate of formation of ultra violet light-induced skin tumors when topically applied to hairless mice 5 days per week for a period of 40 weeks.
Clobetasol propionate was negative in the in vitro mammalian chromosomal aberration test and in the in vivo mammalian erythrocyte micronucleus test.
The effect of subcutaneously administered clobetasol propionate on fertility and general reproductive toxicity was studied in rats at doses of 0, 12.5, 25, and 50 µg/kg/day. Males were treated beginning 70 days before mating and females beginning 15 days before mating through day 7 of gestation. A dosage level of less than 12.5 µg/kg/day clobetasol propionate was considered to be the no-observed-effect-level (NOEL) for paternal and maternal general toxicity based on decreased weight gain and for male reproductive toxicity based on increased weights of the seminal vesicles. The female reproductive NOEL was 12.5 µg/kg/day (ratio of animal dose to proposed human dose of 0.03 on a mg/m2/day basis) based on reduction in the numbers of estrous cycles during the pre-cohabitation period and an increase in the number of nonviable embryos at higher doses.
14 CLINICAL STUDIES
The efficacy of CLOBEX Lotion, 0.05% in psoriasis and atopic dermatitis has been demonstrated in two adequate and well-controlled clinical trials. The first trial was conducted in subjects with moderate to severe plaque psoriasis. Subjects were treated twice daily for 4 weeks with either CLOBEX Lotion, 0.05% or vehicle lotion. Trial results demonstrated that the efficacy of CLOBEX Lotion, 0.05% in treating moderate to severe plaque psoriasis was superior to that of vehicle.
At the end of treatment (4 weeks), 30 of 82 subjects (36.6%) treated with CLOBEX Lotion, 0.05% compared with 0 of 29 (0%) treated with vehicle achieved success. Success was defined as a score of none or very mild (no or very slight clinical signs or symptoms of erythema, plaque elevation, or scaling) on the Global Severity scale of psoriasis.
The second trial was conducted in subjects with moderate to severe atopic dermatitis. Subjects were treated twice daily for 2 weeks with either CLOBEX Lotion, 0.05% or vehicle lotion. Trial results demonstrated that the efficacy of CLOBEX Lotion, 0.05% in treating moderate to severe atopic dermatitis was superior to that of vehicle.
At the end of treatment (2 weeks), 41 of 96 subjects (42.7%) treated with CLOBEX Lotion, 0.05% compared with 4 of 33 (12.1%) treated with vehicle achieved success. Success was defined as a score of none or very mild (no or very slight clinical signs or symptoms of erythema, induration/papulation, oozing/crusting, or pruritus) on the Global Severity scale of atopic dermatitis.
16 HOW SUPPLIED/STORAGE AND HANDLING
CLOBEX Lotion, 0.05% is a white liquid, supplied in the following sizes:
2 fl oz/59 mL NDC 0299-3848-02 high density polyethylene bottles
4 fl oz/118 mL NDC 0299-3848-04 high density polyethylene bottles
Store at USP controlled room temperature 68° to 77°F (20°-25°C), with excursions permitted between 59° and 86°F (15° - 30°C). Protect from freezing.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
Information for Patients
Inform the patient using toical corticosteroids to adhere to the following instructions:
- This medication is to be used as directed by the physician and should not be used longer than the prescribed time period.
- This medication should not be used for any disorder other than that for which it was prescribed.
- Do not use other corticosteroid-containing products while using CLOBEX (clobetasol propionate) Lotion, 0.05%.
- The treated skin area should not be bandaged, otherwise covered, or wrapped so as to be occlusive unless directed by the physician.
- Patients should wash their hands after applying the medication.
- Patients should report any signs of local or systemic adverse reactions to the physician.
- Patients should inform their physicians that they are using CLOBEX (clobetasol propionate) Lotion, 0.05% if surgery is contemplated.
- This medication is for external use only. It should not be used on the face, underarms, or groin area, and avoid contact with the eyes and lips.
- As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, contact the physician.
- Patients should be informed to not use more than 50 g (50 mL or 1.75 fl. oz.) per week of CLOBEX Lotion, 0.05%.
Patient Information
CLOBEX® (KLO-bex)
(clobetasol propionate)
Lotion
Important: For use on skin only. Do not get CLOBEX Lotion near or in your eyes, mouth or vagina.
Read the Patient Information that comes with CLOBEX Lotion before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What is CLOBEX Lotion?
CLOBEX Lotion is a prescription corticosteroid medicine used to reduce the swelling (inflammation) and itching caused by certain skin conditions called corticosteroid-responsive dermatoses, including atopic dermatitis and psoriasis, in people 18 years of age and older. CLOBEX Lotion is for use on the skin only (topical).
- CLOBEX Lotion should only be used for the shortest amount of time needed to treat your skin condition.
- CLOBEX Lotion should not be used for more than 2 weeks in a row unless your doctor tells you to use it for a longer time.
- You should not apply more than 50 mL (1.75 fluid ounces) of CLOBEX Lotion to your skin in 1 week.
You should not use CLOBEX Lotion:
- on your face, underarms (armpits), or groin areas
- if you have thinning of the skin (atrophy) at the treatment site
- to treat rosacea or a rash around your mouth (perioral dermatitis)
CLOBEX Lotion should not be used in children under 18 years of age.
What should I tell my doctor before using CLOBEX Lotion?
Before you use CLOBEX Lotion, tell your doctor if you:
- have a skin infection. You may need medicine to treat the skin infection before you use CLOBEX Lotion.
- have any open sores or cuts on your skin
- plan to have surgery
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if CLOBEX Lotion can harm your unborn baby.
- are breast-feeding or plan to breast-feed. It is not known if CLOBEX Lotion passes into your breast milk. Talk to your doctor about the best way to feed your baby if you use CLOBEX Lotion.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Especially tell your doctor if you take other corticosteroid medicines by mouth or use other products on your skin that contain corticosteroids. You should not use other products that contain corticosteroids while you are using CLOBEX Lotion. Ask your doctor or pharmacist if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use CLOBEX Lotion?
- Use CLOBEX Lotion exactly as your doctor tells you to use it.
- Your doctor should tell you how much CLOBEX Lotion to use and where to apply it.
- CLOBEX Lotion is for skin use only (topical).
- You should not use CLOBEX Lotion on your face, underarms or groin. Avoid getting CLOBEX Lotion in your eyes or on your lips.
- Apply CLOBEX Lotion 2 times each day.
- Apply only enough CLOBEX Lotion to cover your affected skin areas.
To apply CLOBEX Lotion:
- Turn the bottle of CLOBEX Lotion upside down.
- Apply CLOBEX Lotion onto your fingertips or directly on your affected skin area.
- Rub CLOBEX Lotion into your affected skin area gently and completely.
- Repeat these steps to apply CLOBEX Lotion to all affected skin areas as your doctor tells you.
- Wash your hands after applying CLOBEX Lotion.
- Do not bandage, cover or wrap your treated areas unless your doctor tells you to.
- Tell your doctor if your skin condition is not getting better after using CLOBEX Lotion for 2 weeks in a row. Do not use CLOBEX Lotion for more than 2 weeks unless your doctor tells you to.
What are the possible side effects of CLOBEX Lotion?
CLOBEX Lotion can pass through your skin. Too much CLOBEX Lotion passing through your skin can cause your adrenal glands to stop working. Your doctor may do blood tests to check how well your adrenal glands are working.
The most common side effects of CLOBEX Lotion include:
- burning, stinging, itching, redness, irritation and dry skin
- thinning of the skin
- widening of small blood vessels in the skin
- skin discomfort at the site of application
If you go to another doctor for illness, injury or surgery tell your doctor that you are using CLOBEX Lotion.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of CLOBEX Lotion. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Galderma Laboratories, L.P. at 1-866-735-4137.
How should I store CLOBEX Lotion?
- Store CLOBEX Lotion at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze CLOBEX Lotion.
Keep CLOBEX Lotion and all medicines out of reach of children.
General information about CLOBEX Lotion.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CLOBEX Lotion for a condition for which it was not prescribed. Do not give CLOBEX Lotion to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about CLOBEX Lotion. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about CLOBEX Lotion that is written for health professionals.
What are the ingredients in CLOBEX Lotion?
Active ingredient: clobetasol propionate
Inactive ingredients: hypromellose, propylene glycol, mineral oil, polyoxyethylene glycol 300 isostearate, carbomer 1342, sodium hydroxide and purified water.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Marketed by:
GALDERMA LABORATORIES, L.P.
Fort Worth, Texas 76177 USA
Made in Canada
P53324-0
Revised 03/2017
