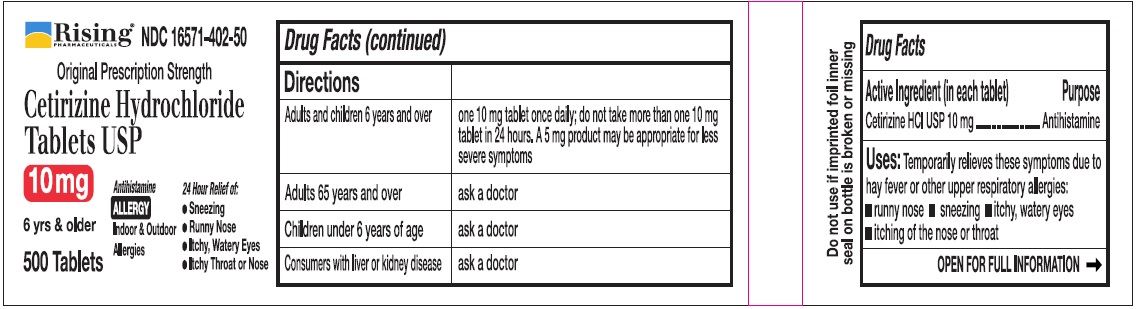
Uses:
Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
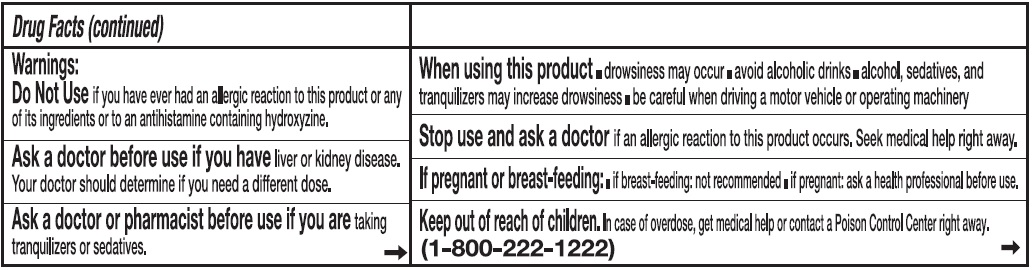
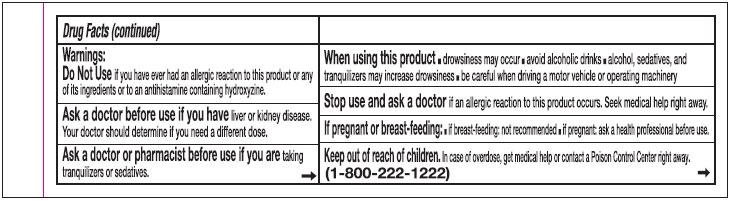
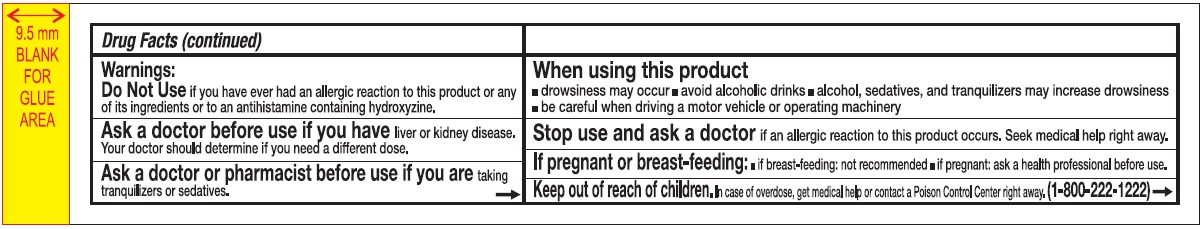
Warnings:
Do Not Use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsines may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinary.
Stop use and ask a doctor if an allergic reaction tothis product occurs. Seek medical help right away.
If pregnant or breast-feeding:
- if breast-feeding: not recommended
- if pregnant: ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
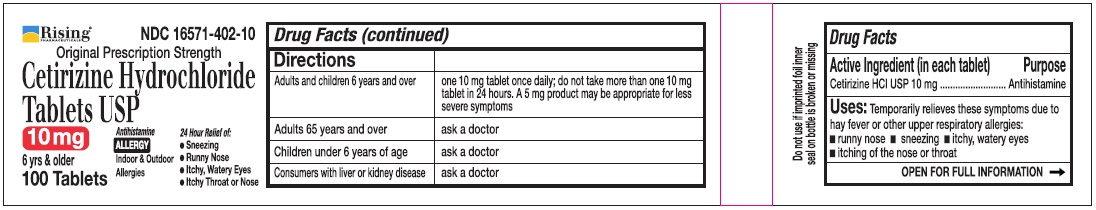
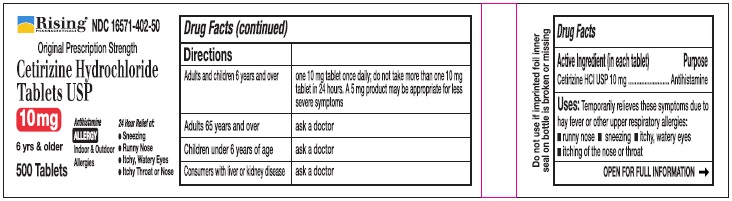
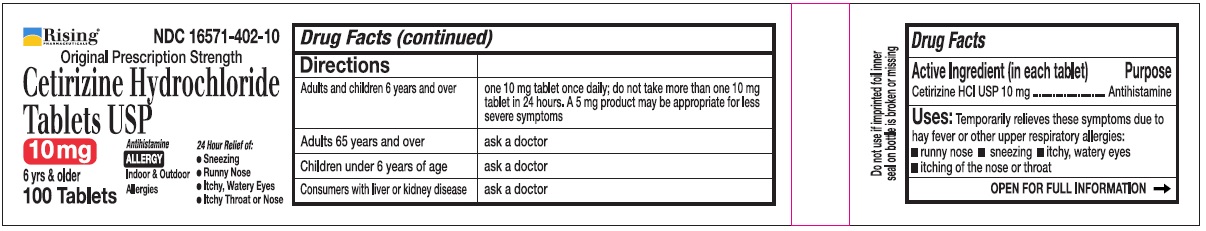
Directions
| Adults and children 6 years and over | one 10 mg tablet once daily, do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms. |
| Adults 65 years and over | Ask a doctor |
| Children under 6 years of age | Ask a doctor |
| Consumers with liver or kidney disease | Ask a doctor |
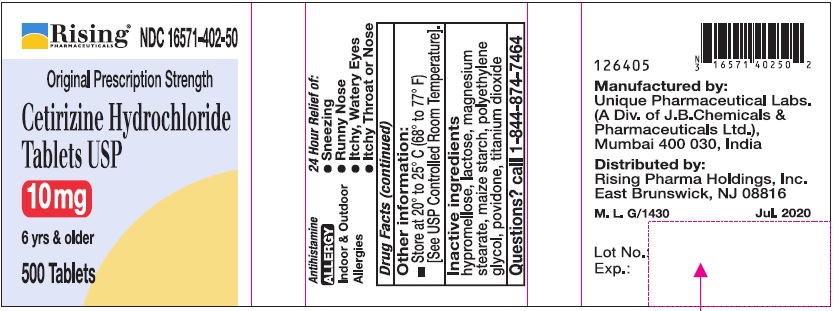
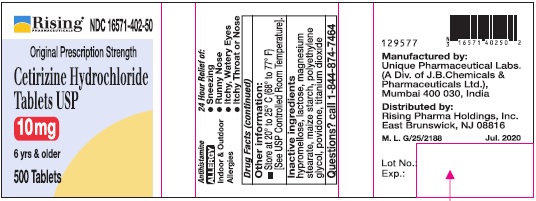
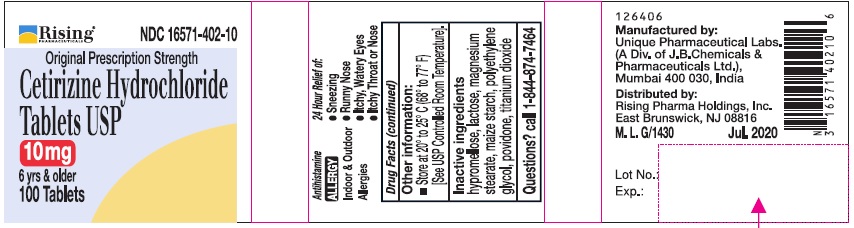
Inactive ingredients
Hypromellose, lactose, magnesium stearate, maize starch, polyethylene glycol, povidone, titanium dioxide.
Manufactured by:
Unique Pharmaceutical Labs.
(A Div. of J. B. Chemicals & Pharmaceuticals Ltd.),
Mumbai 400 030, India
Distributed by:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
M. L. G/1430 Jul. 2020
126406
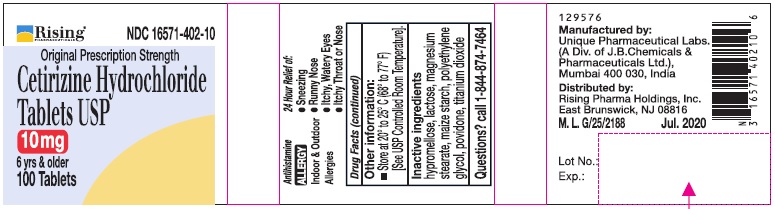
PRINCIPAL DISPLAY PANEL-100'S COUNT
Rising® 16571-402-10
Original Prescription Strength
Cetirizine Hydrochloride Tablets USP 10 mg
6 yrs & older
Antihistamine
ALLERGY
Indoor & Outdoor Allergies
24 Hour Relief of:
•Sneezing
•Runny Nose
•Itchy, Watery Eyes
•Itchy Throat or Nose
100 Tablets
T10 - M.L. G/1430
PRINCIPAL DISPLAY PANEL-500'S COUNT
Rising® 16571-402-50
Original Prescription Strength
Cetirizine Hydrochloride Tablets USP 10 mg
6 yrs & older
Antihistamine
ALLERGY
Indoor & Outdoor Allergies
24 Hour Relief of:
•Sneezing
•Runny Nose
•Itchy, Watery Eyes
•Itchy Throat or Nose
500 Tablets
T10 - M.L. G/1430