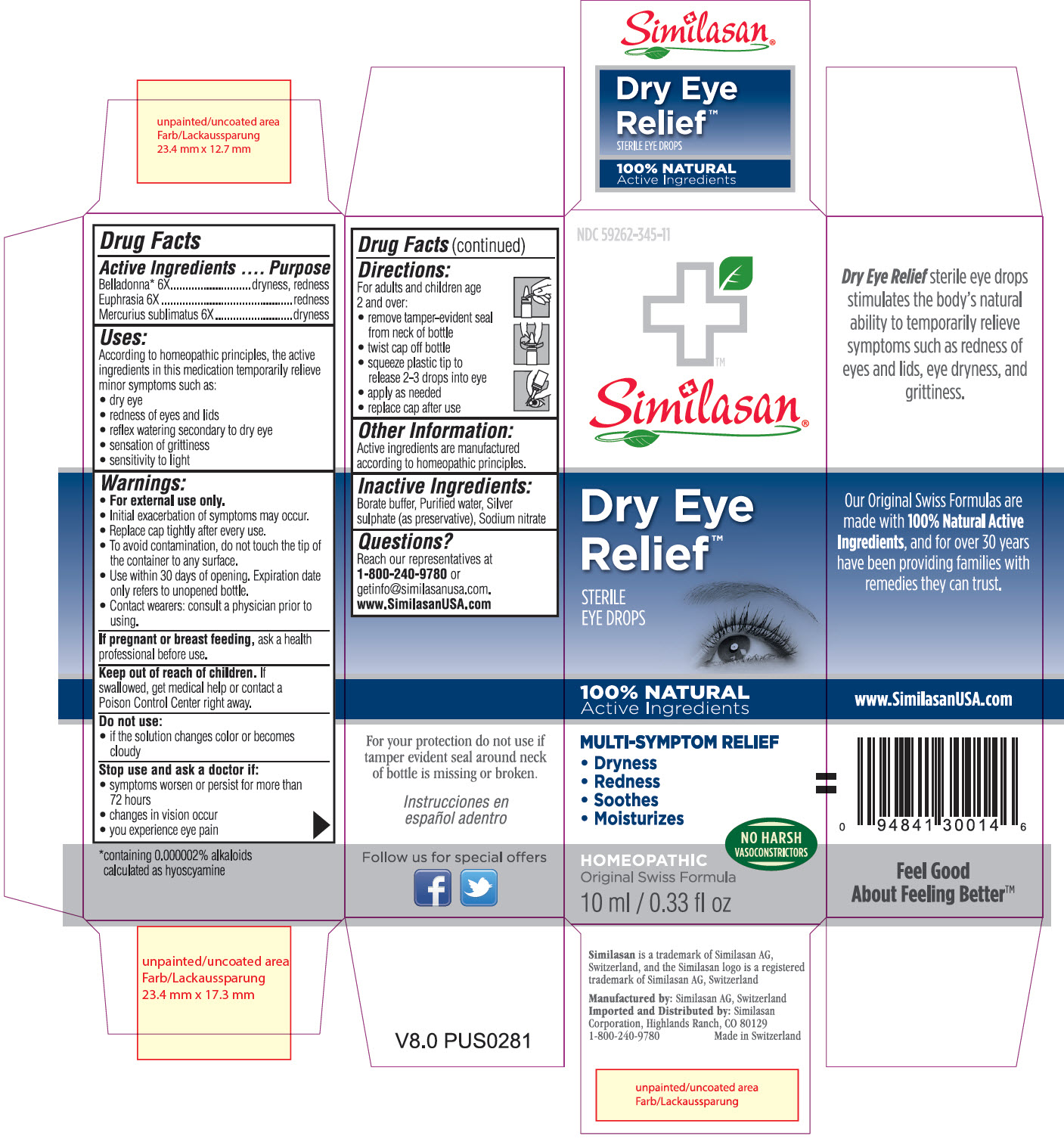
Uses:
According to homeopathic principles, the active ingredients in this medication temporarily relieve minor symptoms such as:
- dry eye
- redness of eyes and lids
- reflex watering secondary to dry eye
- sensation of grittiness
- sensitivity to light
Warnings:
- For external use only.
- Initial exacerbation of symptoms may occur.
- Replace cap tightly after every use.
- To avoid contamination, do not touch the tip of the container to any surface.
- Use within 30 days of opening. Expiration date only refers to unopened bottle.
- Contact wearers: consult a physician prior to using.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if:
- symptoms worsen or persist for more than 72 hours
- changes in vision occur
- you experience eye pain
Directions:
For adults and children age 2 and over:
- remove tamper-evident seal from neck of bottle
- twist cap off bottle
- squeeze plastic tip to release 2-3 drops into eye
- apply as needed
- replace cap after use
Other Information:
Active ingredients are manufactured according to homeopathic principles and are therefore non-toxic and have no known side effects
Inactive Ingredients
Borate buffer, Purified water, Silver sulphate (as preservative), Sodium nitrate