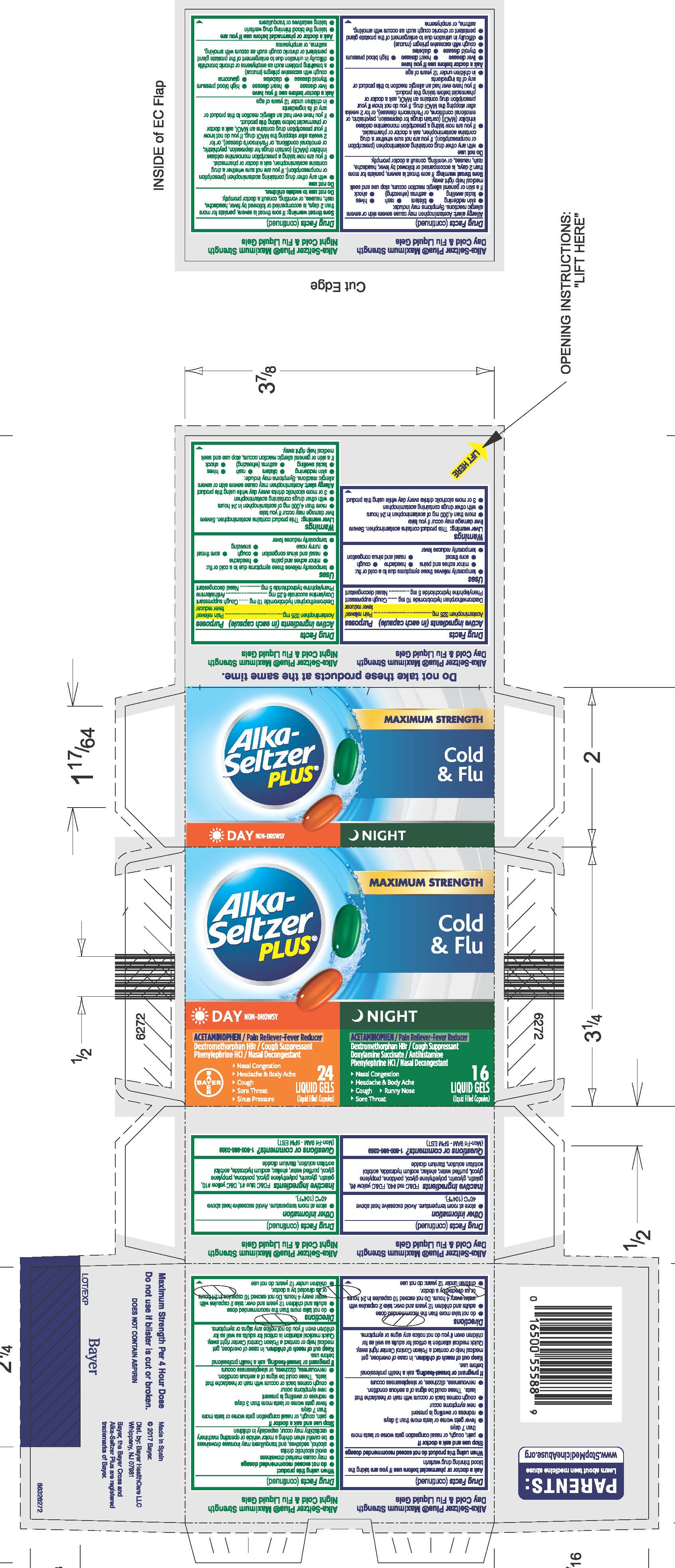
Alka-Seltzer Plus® Maximum Strength Day Cold & Flu Liquid Gels
Alka-Seltzer Plus® Maximum Strength Day Cold & Flu Liquid Gels
Active ingredients (in each capsule)
Acetaminophen 325 mg
Dextromethorphan hydrobromide 10 mg
Phenylephrine hydrochloride 5 mg
Uses
· temporarily relieves these symptoms due to a cold or flu:
· minor aches and pains · headache · cough
· sore throat · nasal and sinus congestion
· temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
· more than 4,000 mg of acetaminophen in 24 hours
· with other drugs containing acetaminophen
· 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin or severe
allergic reactions. Symptoms may include:
· skin reddening · blisters · rash · hives
· facial swelling · asthma (wheezing) · shock
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than
2 days, is accompanied or followed by fever, headache, rash, nausea,
or vomiting, consult a doctor promptly.
Do not use
● with any other drug containing acetaminophen (prescription or
nonprescription). If you are not sure whether a drug contains
acetaminophen, ask a doctor or pharmacist.
● if you are now taking a prescription monoamine oxidase inhibitor
(MAOI) (certain drugs for depression, psychiatric, or emotional
conditions, or Parkinson's disease), or for 2 weeks after stopping
the MAOI drug. If you do not know if your prescription drug contains
an MAOI, ask a doctor or pharmacist before taking this product.
● if you have ever had an allergic reaction to this product or any of its
ingredients
● in children under 12 years of age
Ask a doctor before use if you have
● liver disease ● heart disease ● high blood pressure
● thyroid disease ● diabetes
● cough with excessive phlegm (mucus)
● difficulty in urination due to enlargement of the prostate gland
● persistent or chronic cough such as occurs with smoking, asthma,
or emphysema
When using this product do not exceed recommended dosage
Stop use and ask a doctor if
pain, cough, or nasal congestion gets worse or lasts more than
7 days
· fever gets worse or lasts more than 3 days
· redness or swelling is present
· new symptoms occur
· cough comes back or occurs with rash or headache that lasts.
These could be signs of a serious condition.
· nervousness, dizziness, or sleeplessness occurs
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
Directions
· do not take more than the recommended dose
· adults and children 12 years and over: take 2 capsules with water
every 4 hours. Do not exceed 10 capsules in 24 hours or as
directed by a doctor.
· children under 12 years: do not use
Other information
Other information
● store at room temperature. Avoid excessive heat above 40°C
(104°F).
Inactive ingredients FD&C red #40, FD&C yellow #6, gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, shellac, sodium hydroxide, sorbitol sorbitan solution, titanium dioxide
Active ingredients (in each capsule)
Acetaminophen 325 mg
Dextromethorphan hydrobromide 10 mg
Phenylephrine hydrochloride 5 mg
Uses
· temporarily relieves these symptoms due to a cold or flu:
· minor aches and pains · headache · cough
· sore throat · nasal and sinus congestion
· temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
· more than 4,000 mg of acetaminophen in 24 hours
· with other drugs containing acetaminophen
· 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin or severe
allergic reactions. Symptoms may include:
· skin reddening · blisters · rash · hives
· facial swelling · asthma (wheezing) · shock
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than
2 days, is accompanied or followed by fever, headache, rash, nausea,
or vomiting, consult a doctor promptly.
Do not use
● with any other drug containing acetaminophen (prescription or
nonprescription). If you are not sure whether a drug contains
acetaminophen, ask a doctor or pharmacist.
● if you are now taking a prescription monoamine oxidase inhibitor
(MAOI) (certain drugs for depression, psychiatric, or emotional
conditions, or Parkinson's disease), or for 2 weeks after stopping
the MAOI drug. If you do not know if your prescription drug contains
an MAOI, ask a doctor or pharmacist before taking this product.
● if you have ever had an allergic reaction to this product or any of its
ingredients
● in children under 12 years of age
Ask a doctor before use if you have
● liver disease ● heart disease ● high blood pressure
● thyroid disease ● diabetes
● cough with excessive phlegm (mucus)
● difficulty in urination due to enlargement of the prostate gland
● persistent or chronic cough such as occurs with smoking, asthma,
or emphysema
When using this product do not exceed recommended dosage
Stop use and ask a doctor if
· pain, cough, or nasal congestion gets worse or lasts more than
7 days
· fever gets worse or lasts more than 3 days
· redness or swelling is present
· new symptoms occur
· cough comes back or occurs with rash or headache that lasts.
These could be signs of a serious condition.
· nervousness, dizziness, or sleeplessness occurs
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
· do not take more than the recommended dose
· adults and children 12 years and over: take 2 capsules with water
every 4 hours. Do not exceed 10 capsules in 24 hours or as
directed by a doctor.
· children under 12 years: do not use
Other information
Other information
● store at room temperature. Avoid excessive heat above 40°C
(104°F).
Inactive ingredients FD&C red #40, FD&C yellow #6, gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, shellac, sodium hydroxide, sorbitol sorbitan solution, titanium dioxide
Carton 40 count
Alka-Seltzer PLUS
MAXIMUM STRENGTH
Cold &
Flu
Day NON-DROWSY
ACETAMINOPHEN / Pain Reliever-Fever Reducer
Dextromethorphan HBr / Cough Suppressant
Phenylephrine HCl / Nasal Decongestant
- Nasal Congestion
- Headache & Body Ache
- Cough
- Sore Throat
- Sinus Pressure
24 LIQUID GELS
(Liquid Filled Capsules)
NIGHT
ACETAMINOPHEN / Pain Reliever-Fever Reducer
Dextromethorphan HBr / Cough Suppressant
Doxylamine Succinate / Antihistamine
Phenylephrine HCl / Nasal Decongestant
- Nasal Congestion
- Headache & Body Ache
- Cough
- Runny Nose
- Sore Throat
16 LIQUID GELS
(Liquid Filled Capsules)