FIRSTCARE CHILDRENS ALLERGY RELIEF- diphenhydramine hcl bar, chewable
USpharma Ltd
----------
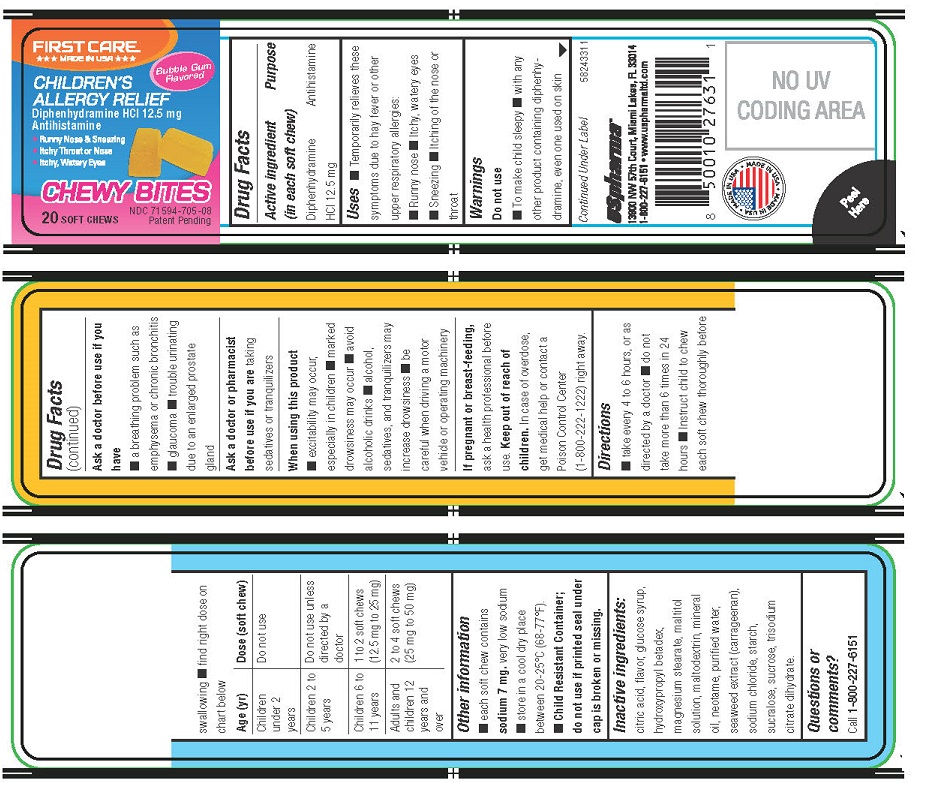
FIRSTCARE CHILDREN'S ALLERGY RELIEF
Uses
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- Runny nose
- Itchy, watery eyes
- Sneezing
- Itching of the nose or throat
Warnings
Do not use
- To make child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
- Instruct child to chew each soft chew thoroughly before swallowing
- find right dose on chart below
Age (yr)
Dose (soft chew)
Children under 2 years
Do not use
Children 2 to 5 years
Do not use unless directed by a doctor
Children 6 to 11 years
1 to 2 soft chews (12.5 mg to 25 mg)
Adults and children 12 years and over
2 to 4 soft chews (25 mg to 50 mg)
Other information
- each soft chew contains sodium 7 mg.
very low sodium
- store in a cool dry place between 20-25°C (68-77°F).
- Child Resistant Container;do not use if printed seal under cap is broken or missing.
| FIRSTCARE CHILDRENS ALLERGY RELIEF
diphenhydramine hcl bar, chewable |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - USpharma Ltd (080664601) |
| Registrant - USpharma Ltd (080664601) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| USpharma Ltd | 080664601 | manufacture(71594-705) , pack(71594-705) | |
Revised: 1/2024
Document Id: 0ec6abde-d6f9-7bda-e063-6394a90ab962
Set id: 540c38ec-3787-4a27-8c5e-5401e3960e20
Version: 3
Effective Time: 20240112
USpharma Ltd