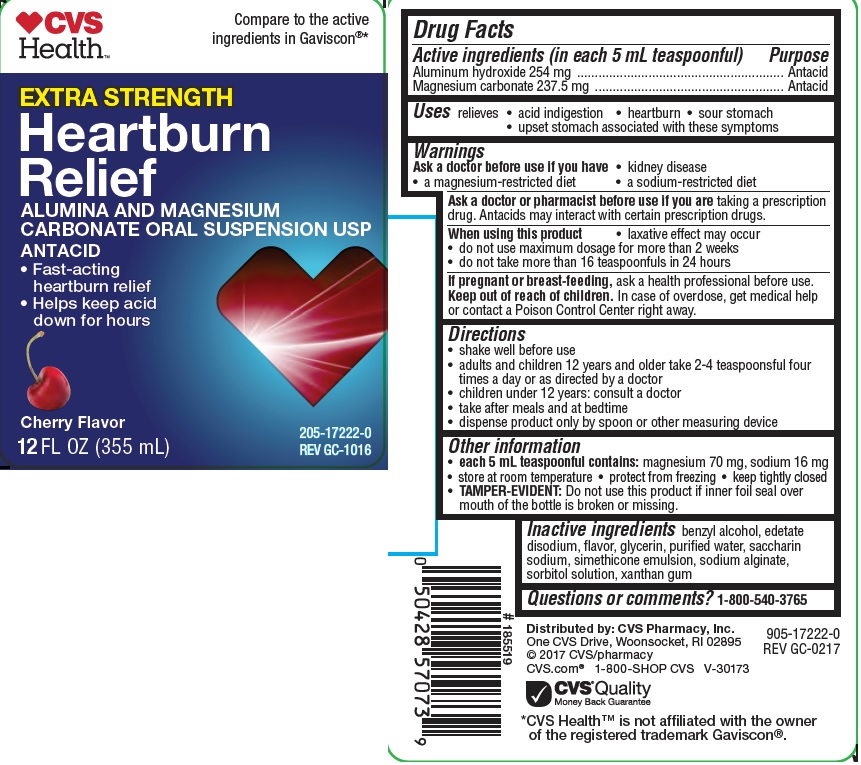
Active ingredients (in each 5 mL teaspoonful)
Aluminum hydroxide 254 mg
Magnesium carbonate 237.5 mg
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium-restricted diet
- a sodium-restricted diet
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- laxative effect may occur
- do not use maximum dosage for more than 2 weeks
- do not take more than 16 teaspoonfuls in 24 hours
If pregnant or breast-feeding, ask a health professional before use.
Directions
- shake well before use
- adults and children 12 years and older take 2-4 teaspoonsful four times a day or as directed by a doctor
- children under 12 years: consult a doctor
- take after meals and at bedtime
- dispense product only by spoon or other measuring device
Other information
- each 5 mL teaspoonful contains: magnesium 70 mg, sodium 16 mg
- store at room temperature
- protect from freezing
- keep tightly closed
- TAMPER-EVIDENT: Do not use this product if inner foil seal over mouth of the bottle is broken or missing.