FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
BYETTA is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus [see Clinical Studies (14)].
Limitations of Use
- •
- BYETTA is not indicated for use in patients with type 1 diabetes.
- •
- BYETTA contains exenatide and should not be used with other products containing the active ingredient exenatide.
- •
- BYETTA has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis [see Warnings and Precautions (5.2)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
- •
- Initiate BYETTA at 5 mcg administered subcutaneously twice daily at any time within the 60-minute period before the morning and evening meals (or before the two main meals of the day, approximately 6 hours or more apart). Do not administer after a meal.
- •
- Based on clinical response, the dose of BYETTA can be increased to 10 mcg twice daily after 1 month of therapy.
- •
- Administer as a subcutaneous injection in the thigh, abdomen, or upper arm.
- •
- Inspect visually for particulate matter and discoloration. Only use BYETTA if the solution appears clear, colorless, and contains no particles.
- •
- Do not mix BYETTA with insulin. Do not transfer BYETTA from the pen to a syringe or a vial.
- •
- If a dose is missed, resume the treatment regimen as prescribed with the next scheduled dose.
3 DOSAGE FORMS AND STRENGTHS
BYETTA injection is a clear, colorless solution of 250 mcg/mL exenatide supplied as follows:
- •
- 5 mcg per dose in a 1.2 mL single-patient-use prefilled pen (60 doses)
- •
- 10 mcg per dose in a 2.4 mL single-patient-use prefilled pen (60 doses)
4 CONTRAINDICATIONS
BYETTA is contraindicated in patients with:
- •
- A prior severe hypersensitivity reaction to exenatide or to any of the excipients in BYETTA. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with BYETTA [see Warnings and Precautions (5.7)].
- •
- A history of drug-induced immune-mediated thrombocytopenia from exenatide products. Serious bleeding, which may be fatal, from drug-induced immune-mediated thrombocytopenia has been reported with exenatide use [see Warnings and Precautions (5.8)].
5 WARNINGS AND PRECAUTIONS
5.1 Never Share a BYETTA Pen Between Patients
BYETTA pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens.
5.2 Acute Pancreatitis
Based on postmarketing data, BYETTA has been associated with acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis. After initiation of BYETTA, and after dose increases, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back, which may or may not be accompanied by vomiting). If pancreatitis is suspected, BYETTA should promptly be discontinued and appropriate management should be initiated. If pancreatitis is confirmed, BYETTA should not be restarted. Consider antidiabetic therapies other than BYETTA in patients with a history of pancreatitis.
5.3 Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
Patients receiving BYETTA in combination with an insulin secretagogue (e.g., sulfonylurea) or insulin may have an increased risk of hypoglycemia including severe hypoglycemia [see Adverse Reactions (6) and Drug Interactions (7.2)].
The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
5.4 Acute Kidney Injury
There have been postmarketing reports of altered renal function with BYETTA, including increased serum creatinine, renal impairment, worsened chronic renal failure, and acute renal failure, sometimes requiring hemodialysis or kidney transplantation. Some of these events occurred in patients receiving one or more pharmacologic agents known to affect renal function or hydration status, such as angiotensin converting enzyme inhibitors, nonsteroidal anti-inflammatory drugs, or diuretics. Some events occurred in patients who had been experiencing nausea, vomiting, or diarrhea, with or without dehydration. Reversibility of altered renal function has been observed in many cases with supportive treatment and discontinuation of potentially causative agents, including BYETTA. Exenatide has not been found to be directly nephrotoxic in preclinical or clinical studies.
BYETTA is not recommended in patients with severe renal impairment (creatinine clearance <30 mL/min) or end-stage renal disease and should be used with caution in patients with renal transplantation [see Use in Specific Populations (8.6)]. Because BYETTA may induce nausea and vomiting with transient hypovolemia, treatment may worsen renal function. Caution should be applied when initiating or escalating doses of BYETTA from 5 to 10 mcg in patients with moderate renal impairment (creatinine clearance 30-50 mL/min).
5.5 Gastrointestinal Disease
BYETTA has not been studied in patients with severe gastrointestinal disease, including gastroparesis. Because BYETTA is commonly associated with gastrointestinal adverse reactions, including nausea, vomiting, and diarrhea, the use of BYETTA is not recommended in patients with severe gastrointestinal disease.
5.6 Immunogenicity
Patients may develop antibodies to exenatide following treatment with BYETTA. Antibody levels were measured in 90% of subjects in the 30-week, 24-week, and 16-week placebo-controlled studies and the 30-week comparator-controlled study of BYETTA. In 3%, 4%, 1%, and 1% of these patients, respectively, antibody formation was associated with an attenuated glycemic response. If there is worsening glycemic control or failure to achieve targeted glycemic control, alternative antidiabetic therapy should be considered [see Adverse Reactions (6.1)].
5.7 Hypersensitivity
There have been postmarketing reports of serious hypersensitivity reactions (e.g., anaphylaxis and angioedema) in patients treated with BYETTA. If a hypersensitivity reaction occurs, the patient should discontinue BYETTA and other suspect medications and promptly seek medical advice. Inform and closely monitor patients with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist for allergic reactions, because it is unknown whether such patients will be predisposed to anaphylaxis with BYETTA [see Adverse Reactions (6.2)].
5.8 Drug-Induced Thrombocytopenia
Serious bleeding, which may be fatal, from drug-induced immune-mediated thrombocytopenia has been reported in the postmarketing setting with exenatide use. Drug-induced thrombocytopenia is an immune-mediated reaction, with exenatide-dependent anti-platelet antibodies. In the presence of exenatide, these antibodies cause platelet destruction. If drug-induced thrombocytopenia is suspected, discontinue BYETTA immediately and do not re-expose the patient to exenatide [see Adverse Reactions (6.2)].
5.9 Acute Gallbladder Disease
Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In a clinical study with exenatide, 1.9% of exenatide-treated patients and 1.4% of placebo-treated patients reported an acute event of gallbladder disease, such as cholelithiasis or cholecystitis. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated.
6 ADVERSE REACTIONS
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- •
- Never Share a BYETTA Pen Between Patients [see Warnings and Precautions (5.1)]
- •
- Acute Pancreatitis [see Warnings and Precautions (5.2)]
- •
- Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin [see Warnings and Precautions (5.3)]
- •
- Acute Kidney Injury [see Warnings and Precautions (5.4)]
- •
- Gastrointestinal Disease [see Warnings and Precautions (5.5)]
- •
- Immunogenicity [see Warnings and Precautions (5.6)]
- •
- Hypersensitivity [see Warnings and Precautions (5.7)]
- •
- Drug-Induced Thrombocytopenia [see Warnings and Precautions (5.8)]
- •
- Acute Gallbladder Disease [see Warnings and Precautions (5.9)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Hypoglycemia
Table 1 summarizes the incidence and rate of hypoglycemia with BYETTA in six placebo-controlled clinical trials.
|
|||
|
Placebo
|
BYETTA
|
BYETTA
|
|
|
Monotherapy (24 Weeks) |
|||
|
N |
77 |
77 |
78 |
|
% Overall |
1.3% |
5.2% |
3.8% |
|
Rate (episodes/patient-year) |
0.03 |
0.21 |
0.52 |
|
% Severe |
0.0% |
0.0% |
0.0% |
|
With Metformin (30 Weeks) |
|||
|
N |
113 |
110 |
113 |
|
% Overall |
5.3% |
4.5% |
5.3% |
|
Rate (episodes/patient-year) |
0.12 |
0.13 |
0.12 |
|
% Severe |
0.0% |
0.0% |
0.0% |
|
With a Sulfonylurea (30 Weeks) |
|||
|
N |
123 |
125 |
129 |
|
% Overall |
3.3% |
14.4% |
35.7% |
|
Rate (episodes/patient-year) |
0.07 |
0.64 |
1.61 |
|
% Severe |
0.0% |
0.0% |
0.0% |
|
With Metformin and a Sulfonylurea (30 Weeks) |
|||
|
N |
247 |
245 |
241 |
|
% Overall |
12.6% |
19.2% |
27.8% |
|
Rate (episodes/patient-year) |
0.58 |
0.78 |
1.71 |
|
% Severe |
0.0% |
0.4% |
0.0% |
|
With a Thiazolidinedione (16 Weeks) |
|||
|
N |
112 |
not evaluated |
121 |
|
% Overall |
7.1% |
not evaluated |
10.7% |
|
Rate (episodes/patient-years) |
0.56 |
not evaluated |
0.98 |
|
% Severe |
0.0% |
not evaluated |
0.0% |
|
With Insulin Glargine with or without Metformin and/or Thiazolidinedione (30 Weeks)† |
|||
|
N |
122 |
not evaluated |
137 |
|
% Overall |
29.5% |
not evaluated |
24.8% |
|
Rate (episodes/patient-years) |
1.58 |
not evaluated |
1.61 |
|
% Severe |
0.8% |
not evaluated |
0.0% |
|
N = number of Intent-to-Treat subjects in each treatment group. |
|||
Immunogenicity
Antibodies were assessed in 90% of subjects in the 30-week, 24-week, and 16-week studies of BYETTA. In the 30-week controlled trials of BYETTA add-on to metformin and/or sulfonylurea, antibodies were assessed at 2- to 6-week intervals. The mean antibody titer peaked at Week 6 and was reduced by 55% by Week 30. Three hundred and sixty patients (38%) had low titer antibodies (<625) to exenatide at 30 weeks. The level of glycemic control (HbA1c) in these patients was generally comparable to that observed in the 534 patients (56%) without antibody titers. An additional 59 patients (6%) had higher titer antibodies (≥625) at 30 weeks. Of these patients, 32 (3% overall) had an attenuated glycemic response to BYETTA; the remaining 27 (3% overall) had a glycemic response comparable to that of patients without antibodies.
In the 16-week trial of BYETTA add-on to thiazolidinediones, with or without metformin, 36 patients (31%) had low titer antibodies to exenatide at 16 weeks. The level of glycemic control in these patients was generally comparable to that observed in the 69 patients (60%) without antibody titer. An additional 10 patients (9%) had higher titer antibodies at 16 weeks. Of these patients, 4 (4% overall) had an attenuated glycemic response to BYETTA; the remaining 6 (5% overall) had a glycemic response comparable to that of patients without antibodies.
In the 24-week trial of BYETTA used as monotherapy, 40 patients (28%) had low titer antibodies to exenatide at 24 weeks. The level of glycemic control in these patients was generally comparable to that observed in the 101 patients (70%) without antibody titers. An additional 3 patients (2%) had higher titer antibodies at 24 weeks. Of these patients, 1 (1% overall) had an attenuated glycemic response to BYETTA; the remaining 2 (1% overall) had a glycemic response comparable to that of patients without antibodies.
Antibodies to exenatide were not assessed in the 30-week placebo-controlled trial of BYETTA used in combination with insulin glargine.
In the 30-week comparator-controlled trial of BYETTA used in combination with insulin glargine and metformin, 60 patients (20%) had low titer antibodies to exenatide at 30 weeks. The level of glycemic control in these patients was generally comparable to that observed in the 234 patients (77%) without antibody titers. An additional 10 patients (3%) had higher titer antibodies at 30 weeks. Of these patients, 2 (1% overall) had an attenuated glycemic response to BYETTA; the remaining 8 (3% overall) had a glycemic response comparable to that of patients without antibodies.
Two hundred and ten patients with antibodies to exenatide in the BYETTA clinical trials were tested for the presence of cross-reactive antibodies to GLP-1 and/or glucagon. No treatment-emergent cross-reactive antibodies were observed across the range of titers.
Other Adverse Reactions
Monotherapy
For the 24-week placebo-controlled study of BYETTA used as a monotherapy, Table 2 summarizes adverse reactions (excluding hypoglycemia) occurring with an incidence ≥2% and occurring more frequently in BYETTA-treated patients compared with placebo-treated patients.
| Monotherapy | Placebo BID
N=77 % | All BYETTA BID
N=155 % |
|---|---|---|
|
||
|
Nausea |
0 |
8 |
|
Vomiting |
0 |
4 |
|
Dyspepsia |
0 |
3 |
|
BID = twice daily. |
||
Adverse reactions reported in ≥1.0% to <2.0% of patients receiving BYETTA and reported more frequently than with placebo included decreased appetite, diarrhea, and dizziness. The most frequently reported adverse reaction associated with BYETTA, nausea, occurred in a dose-dependent fashion.
Two of the 155 patients treated with BYETTA withdrew due to adverse reactions of headache and nausea. No placebo-treated patients withdrew due to adverse reactions.
Cholelithiasis and cholecystitis
In a clinical study with exenatide, 1.9% of exenatide-treated patients and 1.4% of placebo-treated patients reported an acute event of gallbladder disease, such as cholelithiasis or cholecystitis.
Combination Therapy
Add-On to Metformin and/or Sulfonylurea
In the three 30-week controlled trials of BYETTA add-on to metformin and/or sulfonylurea, adverse reactions (excluding hypoglycemia) with an incidence ≥2% and occurring more frequently in BYETTA-treated patients compared with placebo-treated patients are summarized in Table 3.
| Placebo BID
N=483 % | All BYETTA BID
N=963 % |
|
|---|---|---|
|
||
|
Nausea |
18 |
44 |
|
Vomiting |
4 |
13 |
|
Diarrhea |
6 |
13 |
|
Feeling Jittery |
4 |
9 |
|
Dizziness |
6 |
9 |
|
Headache |
6 |
9 |
|
Dyspepsia |
3 |
6 |
|
Asthenia |
2 |
4 |
|
Gastroesophageal Reflux Disease |
1 |
3 |
|
Hyperhidrosis |
1 |
3 |
|
BID = twice daily |
||
Adverse reactions reported in ≥1.0% to <2.0% of patients receiving BYETTA and reported more frequently than with placebo included decreased appetite. Nausea was the most frequently reported adverse reaction and occurred in a dose-dependent fashion. With continued therapy, the frequency and severity decreased over time in most of the patients who initially experienced nausea. Patients in the long-term uncontrolled open-label extension studies at 52 weeks reported no new types of adverse reactions than those observed in the 30-week controlled trials.
The most common adverse reactions leading to withdrawal for BYETTA-treated patients were nausea (3% of patients) and vomiting (1%). For placebo-treated patients, <1% withdrew due to nausea and none due to vomiting.
Add-On to Thiazolidinedione with or without Metformin
For the 16-week placebo-controlled study of BYETTA add-on to a thiazolidinedione, with or without metformin, Table 4 summarizes the adverse reactions (excluding hypoglycemia) with an incidence of ≥2% and occurring more frequently in BYETTA-treated patients compared with placebo-treated patients.
| With a TZD or TZD/MET | Placebo
N=112 % | All BYETTA BID
N=121 % |
|---|---|---|
|
||
|
Nausea |
15 |
40 |
|
Vomiting |
1 |
13 |
|
Dyspepsia |
1 |
7 |
|
Diarrhea |
3 |
6 |
|
Gastroesophageal Reflux Disease |
0 |
3 |
|
BID = twice daily. |
||
Adverse reactions reported in ≥1.0% to <2.0% of patients receiving BYETTA and reported more frequently than with placebo included decreased appetite. Chills (n=4) and injection-site reactions (n=2) occurred only in BYETTA-treated patients. The two patients who reported an injection-site reaction had high titers of antibodies to exenatide. Two serious adverse events (chest pain and chronic hypersensitivity pneumonitis) were reported in the BYETTA arm. No serious adverse events were reported in the placebo arm.
The most common adverse reactions leading to withdrawal for BYETTA-treated patients were nausea (9%) and vomiting (5%). For placebo-treated patients, <1% withdrew due to nausea.
Add-On to Insulin Glargine with or without Metformin and/or Thiazolidinedione (Placebo-Controlled)
For the 30-week placebo-controlled study of BYETTA as add-on to insulin glargine with or without oral antihyperglycemic medications, Table 5 summarizes adverse reactions (excluding hypoglycemia) occurring with an incidence ≥2% and occurring more frequently in BYETTA-treated patients compared with placebo-treated patients.
|
||
|
With Insulin Glargine |
Placebo
|
All BYETTA BID
|
|
Nausea |
8 |
41 |
|
Vomiting |
4 |
18 |
|
Diarrhea |
8 |
18 |
|
Headache |
4 |
14 |
|
Constipation |
2 |
10 |
|
Dyspepsia |
2 |
7 |
|
Asthenia |
1 |
5 |
|
Abdominal Distension |
1 |
4 |
|
Decreased Appetite |
0 |
3 |
|
Flatulence |
1 |
2 |
|
Gastroesophageal Reflux Disease |
1 |
2 |
|
BID = twice daily. |
||
The most frequently reported adverse reactions leading to withdrawal for BYETTA-treated patients were nausea (5.1%) and vomiting (2.9%). No placebo-treated patients withdrew due to nausea or vomiting.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported during post-approval use of BYETTA or other exenatide formulations. Because these events are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Allergy/Hypersensitivity: injection-site reactions, generalized pruritus and/or urticaria, macular or papular rash, angioedema, anaphylactic reaction.
Blood and Lymphatic Systems: drug-induced thrombocytopenia.
Drug Interactions: International normalized ratio (INR) increased with concomitant warfarin use sometimes associated with bleeding [see Drug Interactions (7.3)].
Gastrointestinal: nausea, vomiting, and/or diarrhea resulting in dehydration; abdominal distension, abdominal pain, eructation, constipation, flatulence, acute pancreatitis, hemorrhagic and necrotizing pancreatitis sometimes resulting in death [see Indications and Usage (1)].
Hepatobiliary: cholecystitis, cholelithiasis.
Metabolic: Severe hypoglycemia with concomitant use of sulfonylurea or insulin.
Neurologic: dysgeusia; somnolence.
Renal and Urinary Disorders: altered renal function, including increased serum creatinine, renal impairment, worsened chronic renal failure or acute renal failure (sometimes requiring hemodialysis), kidney transplant, and kidney transplant dysfunction.
Skin and Subcutaneous Tissue Disorders: alopecia
7 DRUG INTERACTIONS
7.1 Orally Administered Drugs
The effect of BYETTA to slow gastric emptying can reduce the extent and rate of absorption of orally administered drugs. BYETTA should be used with caution in patients receiving oral medications that have narrow therapeutic index or require rapid gastrointestinal absorption [see Adverse Reactions (6.2)]. For oral medications that are dependent on threshold concentrations for efficacy, such as contraceptives and antibiotics, patients should be advised to take those drugs at least 1 hour before BYETTA injection. If such drugs are to be administered with food, patients should be advised to take them with a meal or snack when BYETTA is not administered [see Clinical Pharmacology (12.3)].
7.2 Concomitant Use with an Insulin Secretagogue (e.g., Sulfonylurea) or with Insulin
When initiating BYETTA, consider reducing the dose of concomitantly administered insulin secretagogues (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.3) and Adverse Reactions (6)].
7.3 Warfarin
There are postmarketing reports of increased INR sometimes associated with bleeding, with concomitant use of warfarin and BYETTA [see Adverse Reactions (6.2)]. In a drug interaction study, BYETTA did not have a significant effect on INR [see Clinical Pharmacology (12.3)]. In patients taking warfarin, prothrombin time should be monitored more frequently after initiation or alteration of BYETTA therapy. Once a stable prothrombin time has been documented, prothrombin times can be monitored at the intervals usually recommended for patients on warfarin.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited data with BYETTA in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations). Based on animal reproduction studies, there may be risks to the fetus from exposure to BYETTA during pregnancy. BYETTA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal reproduction studies identified increased adverse fetal and neonatal outcomes from exposure to exenatide during pregnancy and lactation in association with maternal effects. In mice, exenatide administered during gestation and lactation caused increased neonatal deaths at systemic exposure 3-times the human exposure resulting from the maximum recommended human dose (MRHD) of 20 mcg/day for BYETTA (see Data).
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with an HbA1c >7 and has been reported to be as high as 20-25% in women with HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryofetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Animal Data
In studies evaluating reproduction and development in pregnant mice and rabbits, maternal animals were administered exenatide, the active ingredient in BYETTA, by subcutaneous injection twice a day.
In pregnant mice given 6, 68, 460, or 760 mcg/kg/day exenatide during fetal organogenesis, skeletal variations associated with slowed fetal growth, including changes in number of rib pairs or vertebral ossifications sites, and wavy ribs were observed at 760 mcg/kg/day, a dose that produced maternal toxicity and yielded systemic exposure 390-times the human exposure resulting from the MRHD of BYETTA based on AUC comparison.
In pregnant rabbits given 0.2, 2, 22, 156, or 260 mcg/kg/day exenatide during fetal organogenesis, irregular fetal skeletal ossifications were observed at 2 mcg/kg/day, a dose yielding systemic exposure up to 12-times the human exposure from the MRHD of BYETTA based on AUC comparison.
In maternal mice given 6, 68, or 760 mcg/kg/day exenatide from gestation day 6 through lactation day 20 (weaning), an increased number of neonatal deaths was observed on postpartum days 2 to 4 in dams given 6 mcg/kg/day, a dose yielding a systemic exposure 3-times the human exposure from the MRHD of BYETTA based on AUC comparison.
8.2 Lactation
Risk Summary
There is no information regarding the presence of BYETTA, in human milk, the effects of BYETTA on the breastfed infant, or the effects of BYETTA on milk production. Exenatide was present in the milk of lactating mice. However, due to species-specific differences in lactation physiology, the clinical relevance of these data is not clear (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for BYETTA and any potential adverse effects on the breastfed child from BYETTA or from the underlying maternal condition.
Data
In lactating mice subcutaneously injected twice a day with exenatide, the concentration of exenatide in milk was up to 2.5% of the concentration in maternal plasma.
8.4 Pediatric Use
The Safety and effectiveness of BYETTA have not been established in pediatric patients.
Effectiveness of BYETTA was not demonstrated in a randomized, double-blind, placebo-controlled study conducted in 120 pediatric patients (78 received BYETTA and 42 received placebo) aged 10 to 17 years with type 2 diabetes mellitus.
8.5 Geriatric Use
Population pharmacokinetic analysis of patients ranging from 22 to 73 years of age suggests that age does not influence the pharmacokinetic properties of exenatide [see Clinical Pharmacology (12.3)]. BYETTA was studied in 282 patients 65 years of age or older and in 16 patients 75 years of age or older. No differences in safety or effectiveness were observed between these patients and younger patients. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection in the elderly based on renal function.
8.6 Renal Impairment
BYETTA is not recommended for use in patients with end-stage renal disease or severe renal impairment (creatinine clearance <30 mL/min) and should be used with caution in patients with renal transplantation. In patients with end-stage renal disease receiving dialysis, single doses of BYETTA 5 mcg were not well-tolerated due to gastrointestinal side effects. No dosage adjustment of BYETTA is required in patients with mild renal impairment (creatinine clearance 50-80 mL/min). Caution should be applied when initiating or escalating doses of BYETTA from 5 to 10 mcg in patients with moderate renal impairment (creatinine clearance 30-50 mL/min) [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No pharmacokinetic study has been performed in patients with a diagnosis of acute or chronic hepatic impairment. Because exenatide is cleared primarily by the kidney, hepatic dysfunction is not expected to affect blood concentrations of exenatide [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
In a clinical study of BYETTA, three patients with type 2 diabetes each experienced a single overdose of 100 mcg SC (10-times the maximum recommended dose). Effects of the overdoses included severe nausea, severe vomiting, and rapidly declining blood glucose concentrations. One of the three patients experienced severe hypoglycemia requiring parenteral glucose administration. The three patients recovered without complication. In the event of overdose, appropriate supportive treatment should be initiated according to the patient's clinical signs and symptoms.
11 DESCRIPTION
BYETTA (exenatide) is a synthetic peptide, glucagon-like peptide-1 (GLP-1) receptor agonist, that was originally identified in the lizard Heloderma suspectum.
Exenatide is a 39-amino acid peptide amide. Exenatide has the empirical formula C184H282N50O60S and molecular weight of 4186.6 Daltons. The amino acid sequence for exenatide is shown below.
H-His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2
BYETTA injection is supplied for subcutaneous administration as a sterile, preserved isotonic solution in a glass cartridge that has been assembled in a pen-injector (pen). Each milliliter (mL) contains 250 micrograms (mcg) synthetic exenatide, 2.2 mg metacresol as an antimicrobial preservative, mannitol as a tonicity-adjusting agent, and glacial acetic acid and sodium acetate trihydrate in water for injection as a buffering solution at pH 4.5. Two prefilled pens are available to deliver unit doses of 5 mcg or 10 mcg. Each prefilled pen will deliver 60 doses to provide for 30 days of twice daily administration (BID).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Incretins, such as glucagon-like peptide-1 (GLP-1), enhance glucose-dependent insulin secretion and exhibit other antihyperglycemic actions following their release into the circulation from the gut. BYETTA is a GLP-1 receptor agonist that enhances glucose-dependent insulin secretion by the pancreatic beta-cell, suppresses inappropriately elevated glucagon secretion, and slows gastric emptying.
The amino acid sequence of exenatide partially overlaps that of human GLP-1. Exenatide has been shown to bind and activate the human GLP-1 receptor in vitro. This leads to an increase in both glucose-dependent synthesis of insulin, and in vivo secretion of insulin from pancreatic beta cells, by mechanisms involving cyclic AMP and/or other intracellular signaling pathways.
BYETTA improves glycemic control by reducing fasting and postprandial glucose concentrations in patients with type 2 diabetes through the actions described below.
12.2 Pharmacodynamics
Glucose-Dependent Insulin Secretion
BYETTA has acute effects on pancreatic beta-cell responsiveness to glucose leading to insulin release predominantly in the presence of elevated glucose concentrations. This insulin secretion subsides as blood glucose concentrations decrease and approach euglycemia. However, BYETTA does not impair the normal glucagon response to hypoglycemia.
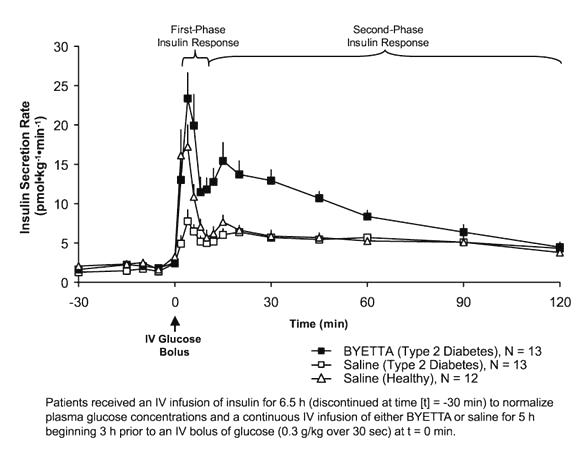
First-Phase Insulin Response
In healthy individuals, robust insulin secretion occurs during the first 10 minutes following intravenous (IV) glucose administration. This secretion, known as the "first-phase insulin response," is characteristically absent in patients with type 2 diabetes. The loss of the first-phase insulin response is an early beta-cell defect in type 2 diabetes. Administration of BYETTA at therapeutic plasma concentrations restored first-phase insulin response to an IV bolus of glucose in patients with type 2 diabetes (Figure 1). Both first-phase insulin secretion and second-phase insulin secretion were significantly increased in patients with type 2 diabetes treated with BYETTA compared with saline (p<0.001 for both).
Figure 1: Mean (+SEM) Insulin Secretion Rate during Infusion of BYETTA or Saline in Patients with Type 2 Diabetes and during Infusion of Saline in Healthy Subjects
Glucagon Secretion
In patients with type 2 diabetes, BYETTA moderates glucagon secretion and lowers serum glucagon concentrations during periods of hyperglycemia. Lower glucagon concentrations lead to decreased hepatic glucose output and decreased insulin demand.
Gastric Emptying
BYETTA slows gastric emptying, thereby reducing the rate at which meal-derived glucose appears in the circulation.
Food Intake
In both animals and humans, administration of exenatide has been shown to reduce food intake.
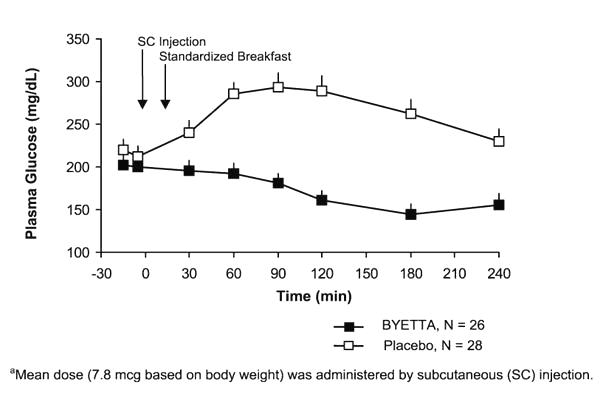
Postprandial Glucose
In patients with type 2 diabetes, BYETTA reduces postprandial plasma glucose concentrations (Figure 2).
Figure 2: Mean (+SEM) Postprandial Plasma Glucose Concentrations on Day 1 of BYETTAa Treatment in Patients with Type 2 Diabetes Treated with Metformin, a Sulfonylurea, or Both (N=54)
Fasting Glucose
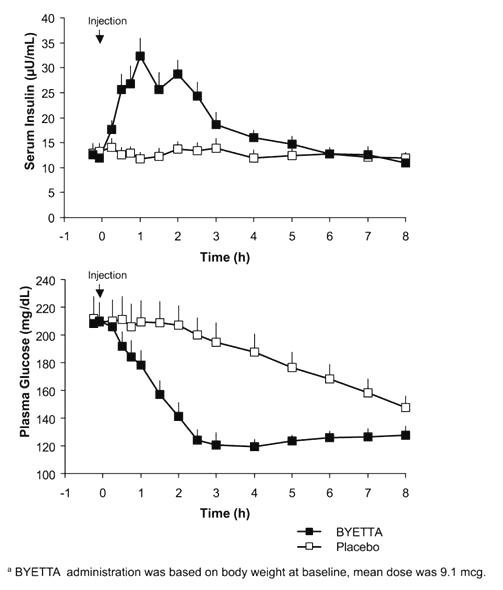
In a single-dose crossover study in patients with type 2 diabetes and fasting hyperglycemia, immediate insulin release followed injection of BYETTA. Plasma glucose concentrations were significantly reduced with BYETTA compared with placebo (Figure 3).
Figure 3: Mean (+SEM) Serum Insulin and Plasma Glucose Concentrations Following a One-Time Injection of BYETTAa or Placebo in Fasting Patients with Type 2 Diabetes (N=12)
Cardiac Electrophysiology
The effect of exenatide 10 µg subcutaneously on QTc interval was evaluated in a randomized, placebo-, and active-controlled (moxifloxacin 400 mg) crossover thorough QTc study in 62 healthy subjects. In this study with demonstrated ability to detect small effects, the upper bound of the 90% confidence interval for the largest placebo-adjusted, baseline-corrected QTc was below 10 msec. Thus, BYETTA (10 mcg single dose) was not associated with clinically meaningful prolongation of the QTc interval.
12.3 Pharmacokinetics
Absorption
Following SC administration to patients with type 2 diabetes, exenatide reaches median peak plasma concentrations in 2.1 hours. The mean peak exenatide concentration (Cmax) was 211 pg/mL and overall mean area under the time-concentration curve (AUC0-inf) was 1036 pg∙h/mL following SC administration of a 10-mcg dose of BYETTA. Exenatide exposure (AUC) increased proportionally over the therapeutic dose range of 5 to 10 mcg. The Cmax values increased less than proportionally over the same range. Similar exposure is achieved with SC administration of BYETTA in the abdomen, thigh, or upper arm.
Distribution
The mean apparent volume of distribution of exenatide following SC administration of a single dose of BYETTA is 28.3 L.
Metabolism and Elimination
Nonclinical studies have shown that exenatide is predominantly eliminated by glomerular filtration with subsequent proteolytic degradation. The mean apparent clearance of exenatide in humans is 9.1 L/hour and the mean terminal half-life is 2.4 hours. These pharmacokinetic characteristics of exenatide are independent of the dose. In most individuals, exenatide concentrations are measurable for approximately 10 hours post-dose.
Drug Interactions
Acetaminophen
When 1000 mg acetaminophen elixir was given with 10 mcg BYETTA (0 hour) and 1 hour, 2 hours, and 4 hours after BYETTA injection, acetaminophen AUCs were decreased by 21%, 23%, 24%, and 14%, respectively; Cmax was decreased by 37%, 56%, 54%, and 41%, respectively; Tmax was increased from 0.6 hour in the control period to 0.9 hour, 4.2 hours, 3.3 hours, and 1.6 hours, respectively. Acetaminophen AUC, Cmax and Tmax were not significantly changed when acetaminophen was given 1 hour before BYETTA injection.
Digoxin
Administration of repeated doses of BYETTA (10 mcg BID) 30 minutes before oral digoxin (0.25 mg once daily) decreased the Cmax of digoxin by 17% and delayed the Tmax of digoxin by approximately 2.5 hours; however, the overall steady-state pharmacokinetic exposure (e.g., AUC) of digoxin was not changed.
Lovastatin
Administration of BYETTA (10 mcg BID) 30 minutes before a single oral dose of lovastatin (40 mg) decreased the AUC and Cmax of lovastatin by approximately 40% and 28%, respectively, and delayed the Tmax by about 4 hours compared with lovastatin administered alone. In the 30-week controlled clinical trials of BYETTA, the use of BYETTA in patients already receiving HMG CoA reductase inhibitors was not associated with consistent changes in lipid profiles compared to baseline.
Lisinopril
In patients with mild to moderate hypertension stabilized on lisinopril (5-20 mg/day), BYETTA (10 mcg BID) did not alter steady-state Cmax or AUC of lisinopril. Lisinopril steady-state Tmax was delayed by 2 hours. There were no changes in 24-hour mean systolic and diastolic blood pressure.
Oral Contraceptives
The effect of BYETTA (10 mcg BID) on single and on multiple doses of a combination oral contraceptive (30 mcg ethinyl estradiol plus 150 mcg levonorgestrel) was studied in healthy female subjects. Repeated daily doses of the oral contraceptive (OC) given 30 minutes after BYETTA administration decreased the Cmax of ethinyl estradiol and levonorgestrel by 45% and 27%, respectively, and delayed the Tmax of ethinyl estradiol and levonorgestrel by 3.0 hours and 3.5 hours, respectively, as compared to the oral contraceptive administered alone. Administration of repeated daily doses of the OC one hour prior to BYETTA administration decreased the mean Cmax of ethinyl estradiol by 15% but the mean Cmax of levonorgestrel was not significantly changed as compared to when the OC was given alone. BYETTA did not alter the mean trough concentrations of levonorgestrel after repeated daily dosing of the oral contraceptive for both regimens. However, the mean trough concentration of ethinyl estradiol was increased by 20% when the OC was administered 30 minutes after BYETTA administration injection as compared to when the OC was given alone. The effect of BYETTA on OC pharmacokinetics is confounded by the possible food effect on OC in this study. Therefore, OC products should be administered at least one hour prior to BYETTA injection.
Warfarin
Administration of warfarin (25 mg) 35 minutes after repeated doses of BYETTA (5 mcg BID on Days 1-2 and 10 mcg BID on Days 3-9) in healthy volunteers delayed warfarin Tmax by approximately 2 hours. No clinically relevant effects on Cmax or AUC of S- and R-enantiomers of warfarin were observed. BYETTA did not significantly alter the pharmacodynamic properties (e.g., international normalized ratio) of warfarin [see Drug Interactions (7.3)].
Specific Populations
Renal Impairment
Pharmacokinetics of exenatide was studied in subjects with normal, mild, or moderate renal impairment and subjects with end-stage renal disease. In subjects with mild to moderate renal impairment (creatinine clearance 30-80 mL/min), exenatide exposure was similar to that of subjects with normal renal function. However, in subjects with end-stage renal disease receiving dialysis, mean exenatide exposure increased by 3.37-fold compared to that of subjects with normal renal function [see Use in Specific Populations (8.6)].
Hepatic Impairment
No pharmacokinetic study has been performed in patients with a diagnosis of acute or chronic hepatic impairment [see Use in Specific Populations (8.7)].
Age
Population pharmacokinetic analysis of patients ranging from 22 to 73 years of age suggests that age does not influence the pharmacokinetic properties of exenatide [see Use in Specific Population (8.5)].
Gender
Population pharmacokinetic analysis of male and female patients suggests that gender does not influence the distribution and elimination of exenatide.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 104-week carcinogenicity study was conducted in male and female rats at doses of 18, 70, or 250 mcg/kg/day administered by bolus SC injection. Benign thyroid C-cell adenomas were observed in female rats at all exenatide doses. The incidences in female rats were 8% and 5% in the two control groups and 14%, 11%, and 23% in the low-, medium-, and high-dose groups with systemic exposures of 5-, 22-, and 130-times, respectively, the human exposure resulting from the maximum recommended dose of 20 mcg/day, based on plasma area under the curve (AUC).
In a 104-week carcinogenicity study in mice at doses of 18, 70, or 250 mcg/kg/day administered by bolus SC injection, no evidence of tumors was observed at doses up to 250 mcg/kg/day, a systemic exposure up to 95-times the human exposure resulting from the maximum recommended dose of 20 mcg/day, based on AUC.
Exenatide was not mutagenic or clastogenic, with or without metabolic activation, in the Ames bacterial mutagenicity assay or chromosomal aberration assay in Chinese hamster ovary cells. Exenatide was negative in the in vivo mouse micronucleus assay.
In mouse fertility studies with SC doses of 6, 68, or 760 mcg/kg/day, males were treated for 4 weeks prior to and throughout mating, and females were treated 2 weeks prior to mating and throughout mating until gestation day 7. No adverse effect on fertility was observed at 760 mcg/kg/day, a systemic exposure 390-times the human exposure resulting from the maximum recommended dose of 20 mcg/day, based on AUC.
14 CLINICAL STUDIES
BYETTA has been studied as monotherapy and in combination with metformin, a sulfonylurea, a thiazolidinedione, a combination of metformin and a sulfonylurea, a combination of metformin and a thiazolidinedione, or in combination with insulin glargine with or without metformin and/or thiazolidinedione.
14.1 Monotherapy
In a randomized, double-blind, placebo-controlled trial of 24 weeks duration, BYETTA 5 mcg BID (n=77), BYETTA 10 mcg BID (n=78), or placebo BID (n=77) was used as monotherapy in patients with entry HbA1c ranging from 6.5% to 10%. All patients assigned to BYETTA initially received 5 mcg BID for 4 weeks. After 4 weeks, those patients either continued to receive BYETTA 5 mcg BID or had their dose increased to 10 mcg BID. Patients assigned to placebo received placebo BID throughout the trial. BYETTA or placebo was injected subcutaneously before the morning and evening meals. The majority of patients (68%) were Caucasian, 26% West Asian, 3% Hispanic, 3% Black, and 0.4% East Asian.
The primary endpoint was the change in HbA1c from baseline to Week 24 (or the last value at time of early discontinuation). Compared to placebo, BYETTA 5 mcg BID and 10 mcg BID resulted in statistically significant reductions in HbA1c from baseline at Week 24 (Table 6).
| Placebo
BID | BYETTA 5 mcg BID | BYETTA 10 mcg*
BID |
|
|---|---|---|---|
|
|||
|
Intent-to-Treat Population (N) |
77 |
77 |
78 |
|
HbA1c (%), Mean |
|||
|
Baseline |
7.8 |
7.9 |
7.8 |
|
Change at Week 24† |
−0.2 |
−0.7 |
−0.9 |
|
Difference from placebo† (95% CI) |
−0.5 [−0.9, −0.2]‡ |
−0.7 [−1.0, −0.3] |
|
|
Proportion Achieving HbA1c <7% |
38% |
48% |
53% |
|
Body Weight (kg), Mean |
|||
|
Baseline |
86.1 |
85.1 |
86.2 |
|
Change at Week 24† |
−1.5 |
−2.7 |
−2.9 |
|
Difference from placebo† (95% CI) |
−1.3 [−2.3, −0.2] |
−1.5 [−2.5, −0.4] |
|
|
Fasting Serum Glucose§ (mg/dL), Mean |
|||
|
Baseline |
159 |
166 |
155 |
|
Change at Week 24† |
−5 |
−17 |
−19 |
|
Difference from placebo† (95% CI) |
−12 [−23.2, −1.3] |
−14 [−24.5, −2.5] |
|
|
BID = twice daily. |
|||
On average, there were no adverse effects of exenatide on blood pressure or lipids.
14.2 Combination Therapy with Oral Antihyperglycemic Medicines
Three 30-week, double-blind, placebo-controlled trials were conducted to evaluate the safety and efficacy of BYETTA in patients with type 2 diabetes whose glycemic control was inadequate with metformin alone, a sulfonylurea alone, or metformin in combination with a sulfonylurea. In addition, a 16-week, placebo-controlled trial was conducted where BYETTA was added to existing thiazolidinedione (pioglitazone or rosiglitazone) treatment, with or without metformin, in patients with type 2 diabetes with inadequate glycemic control.
In the 30-week trials, after a 4-week placebo lead-in period, patients were randomly assigned to receive BYETTA 5 mcg BID, BYETTA 10 mcg BID, or placebo BID before the morning and evening meals, in addition to their existing oral antidiabetic agent. All patients assigned to BYETTA initially received 5 mcg BID for 4 weeks. After 4 weeks, those patients either continued to receive BYETTA 5 mcg BID or had their dose increased to 10 mcg BID. Patients assigned to placebo received placebo BID throughout the study. A total of 1446 patients were randomized in the three 30-week trials: 991 (69%) were Caucasian, 224 (16%) Hispanic, and 174 (12%) Black. Mean HbA1c values at baseline for the trials ranged from 8.2% to 8.7%.
In the placebo-controlled trial of 16 weeks duration, BYETTA (n=121) or placebo (n=112) was added to existing thiazolidinedione (pioglitazone or rosiglitazone) treatment, with or without metformin. Randomization to BYETTA or placebo was stratified based on whether the patients were receiving metformin. BYETTA treatment was initiated at a dose of 5 mcg BID for 4 weeks then increased to 10 mcg BID for 12 more weeks. Patients assigned to placebo received placebo BID throughout the study. BYETTA or placebo was injected subcutaneously before the morning and evening meals. In this trial, 79% of patients were taking a thiazolidinedione and metformin and 21% were taking a thiazolidinedione alone. The majority of patients (84%) were Caucasian, 8% Hispanic, and 3% Black. The mean baseline HbA1c values were 7.9% for BYETTA and placebo.
The primary endpoint in each study was the mean change in HbA1c from baseline to study end (or early discontinuation). Table 7 summarizes the study results for the 30- and 16-week clinical trials.
| Placebo
BID | BYETTA 5 mcg
BID | BYETTA 10 mcg*
BID |
|
|---|---|---|---|
|
|||
|
In Combination with Metformin (30 Weeks) |
|||
|
Intent-to-Treat Population (N) |
113 |
110 |
113 |
|
HbA1c (%), Mean |
|||
|
Baseline |
8.2 |
8.3 |
8.2 |
|
Change at Week 30† |
−0.0 |
−0.5 |
−0.9 |
|
Difference from placebo† (95% CI) |
−0.5 [−0.7, −0.2]‡ |
−0.9 [−1.1, −0.6]‡ |
|
|
Proportion Achieving HbA1c <7% |
12% |
32% |
40% |
|
Body Weight (kg), Mean |
|||
|
Baseline |
99.9 |
100.0 |
100.9 |
|
Change at Week 30† |
−0.2 |
−1.3 |
−2.6 |
|
Difference from placebo† (95% CI) |
−1.1 [−2.2, −0.0] |
−2.4 [−3.5, −1.3] |
|
|
Fasting Plasma Glucose§ (mg/dL), Mean |
|||
|
Baseline |
169 |
176 |
168 |
|
Change at Week 30† |
+14 |
−5 |
−10 |
|
Difference from placebo† (95% CI) |
−20 [−32, −7] |
−24 [−37, −12] |
|
|
In Combination with a Sulfonylurea (30 Weeks) |
|||
|
Intent-to-Treat Population (N) |
123 |
125 |
129 |
|
HbA1c (%), Mean |
|||
|
Baseline |
8.7 |
8.5 |
8.6 |
|
Change at Week 30† |
+0.1 |
−0.5 |
−0.9 |
|
Difference from placebo† (95% CI) |
−0.6 [−0.9, −0.3]‡ |
−1.0 [−1.3, −0.7]‡ |
|
|
Proportion Achieving HbA1c <7% |
10% |
25% |
36% |
|
Body Weight (kg), Mean |
|||
|
Baseline |
99.1 |
94.9 |
95.2 |
|
Change at Week 30† |
−0.8 |
−1.1 |
−1.6 |
|
Difference from placebo† (95% CI) |
−0.3 [−1.1, 0.6] |
−0.9 [−1.7, −0.0] |
|
|
Fasting Plasma Glucose§ (mg/dL), Mean |
|||
|
Baseline |
194 |
180 |
178 |
|
Change at Week 30† |
+6 |
−5 |
−11 |
|
Difference from placebo† (95% CI) |
−11 [−25, 3] |
−17 [−30, −3] |
|
|
In Combination with Metformin and a Sulfonylurea (30 Weeks) |
|||
|
Intent-to-Treat Population (N) |
247 |
245 |
241 |
|
HbA1c (%), Mean | |||
|
Baseline |
8.5 |
8.5 |
8.5 |
|
Change at Week 30† |
+0.1 |
−0.7 |
−0.9 |
|
Difference from placebo† (95% CI) |
−0.8 [−1.0, −0.6]‡ |
−1.0 [−1.2, −0.8]‡ |
|
|
Proportion Achieving HbA1c <7% |
8% |
25% |
31% |
|
Body Weight (kg), Mean | |||
|
Baseline |
99.1 |
96.9 |
98.4 |
|
Change at Week 30† |
−0.9 |
−1.6 |
−1.6 |
|
Difference from placebo† (95% CI) |
−0.7 [−1.2, −0.2] |
−0.7 [−1.3, −0.2] |
|
|
Fasting Plasma Glucose§ (mg/dL), Mean |
|||
|
Baseline |
181 |
182 |
178 |
|
Change at Week 30† |
+13 |
−11 |
−12 |
|
Difference from placebo† (95% CI) |
−24 [−33, −15] |
−25 [−34, −16] |
|
|
In Combination with a Thiazolidinedione or a Thiazolidinedione plus Metformin (16 Weeks) |
|||
|
Intent-to-Treat Population (N) |
112 |
Dose not studied |
121 |
|
HbA1c (%), Mean |
|||
|
Baseline |
7.9 |
Dose not studied |
7.9 |
|
Change at Week 16† |
+0.1 |
Dose not studied |
−0.7 |
|
Difference from placebo† (95% CI) |
Dose not studied |
−0.9 [−1.1, −0.7]‡ |
|
|
Proportion Achieving HbA1c <7% |
15% |
Dose not studied |
51% |
|
Body Weight (kg), Mean |
|||
|
Baseline |
96.8 |
Dose not studied |
97.5 |
|
Change at Week 16† |
−0.0 |
Dose not studied |
−1.5 |
|
Difference from placebo† (95% CI) |
Dose not studied |
−1.5 [−2.2, −0.7] |
|
|
Fasting Serum Glucose§ (mg/dL), Mean |
|||
|
Baseline |
159 |
Dose not studied |
164 |
|
Change at Week 16† |
+4 |
Dose not studied |
−21 |
|
Difference from placebo† (95% CI) |
Dose not studied |
−25 [−33, −16] |
|
|
BID = twice daily. |
|||
HbA1c
The addition of BYETTA to a regimen of metformin, a sulfonylurea, or both, resulted in statistically significant reductions from baseline in HbA1c compared with patients receiving placebo added to these agents in the three controlled trials (Table 7).
In the 16-week trial of BYETTA add-on to thiazolidinediones, with or without metformin, BYETTA resulted in statistically significant reductions from baseline in HbA1c compared with patients receiving placebo (Table 7).
Postprandial Glucose
Postprandial glucose was measured after a mixed meal tolerance test in 9.5% of patients participating in the 30-week add-on to metformin, add-on to sulfonylurea, and add-on to metformin in combination with sulfonylurea clinical trials. In this pooled subset of patients, BYETTA reduced postprandial plasma glucose concentrations in a dose-dependent manner. The mean (SD) change in 2-hour postprandial glucose concentration following administration of BYETTA at Week 30 relative to baseline was −63 (65) mg/dL for 5 mcg BID (n=42), −71 (73) mg/dL for 10 mcg BID (n=52), and +11 (69) mg/dL for placebo BID (n=44).
14.3 Combination with Insulin Glargine
30-Week Placebo-Controlled Trial
A 30-week, double-blind, placebo-controlled trial was conducted to evaluate the efficacy and safety of BYETTA (n=137) versus placebo (n=122) when added to titrated insulin glargine, with or without metformin and/or thiazolidinedione, in patients with type 2 diabetes with inadequate glycemic control.
All patients assigned to BYETTA initially received 5 mcg BID for 4 weeks. After 4 weeks, those patients assigned to BYETTA had their dose increased to 10 mcg BID. Patients assigned to placebo received placebo BID throughout the trial. BYETTA or placebo was injected subcutaneously before the morning and evening meals. Patients with an HbA1c ≤8.0% decreased their prestudy dose of insulin glargine by 20% and patients with an HbA1c ≥8.1% maintained their current dose of insulin glargine. Five weeks after initiating randomized treatment, insulin doses were titrated with guidance from the investigator toward predefined fasting glucose targets according to the dose titration algorithm provided in Table 9. The majority of patients (78%) were Caucasian, 10% American Indian or Alaska Native, 9% Black, 3% Asian, and 0.8% of multiple origins.
The primary endpoint was the change in HbA1c from baseline to Week 30. Compared to placebo, BYETTA 10 mcg BID resulted in statistically significant reductions in HbA1c from baseline at Week 30 (Table 8) in patients receiving titrated insulin glargine.
| Placebo BID
+ Titrated Insulin Glargine | BYETTA 10 mcg* BID
+ Titrated Insulin Glargine |
|
|---|---|---|
|
||
|
Intent-to-Treat Population (N) |
122 |
137 |
|
HbA1c (%), Mean |
||
|
Baseline |
8.5 |
8.3 |
|
Change at Week 30† |
−1.0 |
−1.7 |
|
Difference from placebo† (95% CI) |
−0.7 [−1.0, −0.5]‡ |
|
|
Proportion Achieving HbA1c <7% |
29% |
56% |
|
Body Weight (kg), Mean |
||
|
Baseline |
93.8 |
95.4 |
|
Change at Week 30§ |
1.0 |
−1.8 |
|
Difference from placebo§ (95% CI) |
−2.7 [−3.7, −1.7]‡ |
|
|
Fasting Serum Glucose¶ (mg/dL), Mean |
||
|
Baseline |
133 |
132 |
|
Change at Week 30§ |
−16 |
−23 |
|
Difference from placebo§ (95% CI) |
−7 [−18, 3] |
|
|
BID = twice daily. |
||
|
|
|
Fasting Plasma Glucose Values
|
Dose Change
|
|
<56† |
−4 |
|
56 to 72† |
−2 |
|
73 to 99‡ |
0 |
|
100 to 119‡ |
+2 |
|
120 to 139‡ |
+4 |
|
140 to 179‡ |
+6 |
|
≥180‡ |
+8 |
|
Abbreviations: U = units. |
|
30-Week Comparator-Controlled Noninferiority Trial
A 30 week, open-label, active comparator-controlled, noninferiority study was conducted to evaluate the safety and efficacy of BYETTA (n=315) versus titrated insulin lispro (n=312) on a background of optimized basal insulin glargine and metformin in patients with type 2 diabetes with inadequate glycemic control.
Following a 12-week basal insulin optimization (BIO) phase, subjects with an HbA1c >7.0% entered a 30-week intervention phase and were randomized to add either BYETTA or insulin lispro to their existing regimen of insulin glargine and metformin. Insulin glargine was titrated to a target fasting plasma glucose of 72 to 100 mg/dL.
All patients assigned to BYETTA initially received 5 mcg BID for four weeks. After four weeks, their dose was increased to 10 mcg BID. Patients in the BYETTA-treated arm with an HbA1c ≤8.0% at the end of the BIO phase decreased their insulin glargine dose by at least 10%.
All patients assigned to insulin lispro three times daily (TID) maintained their prior total daily insulin dose at baseline; however, the initial insulin lispro dose was ⅓ to ½ of the total daily insulin dose with the insulin glargine dose reduced accordingly. The insulin lispro dose was titrated based on preprandial glucose values.
The majority of patients (87%) were Caucasian, 7% American Indian or Alaska Native, 5% Asian, and <1% African American.
The primary endpoint was the change in HbA1c from baseline to Week 30. Both BYETTA 10 mcg BID and titrated lispro provided a mean reduction in HbA1c at Week 30 that met the pre-specified non-inferiority margin of 0.4%.
|
||
|
Titrated Insulin Lispro TID + Titrated Insulin Glargine |
BYETTA 10 mcg* BID + Titrated Insulin Glargine |
|
|
Intent-to-Treat Population (N) |
312 |
315 |
|
HbA1c (%), Mean |
||
|
Baseline |
8.2 |
8.3 |
|
−1.1 |
−1.1 |
|
|
−0.0 [−0.2, 0.1] |
||
|
Body Weight (kg), Mean |
||
|
Baseline |
89.3 |
89.9 |
|
1.9 |
−2.6 |
|
|
−4.5 [−5.2, −3.9] |
||
|
Fasting Serum Glucose§(mg/dL), Mean |
||
|
Baseline |
126 |
130 |
|
5 |
−7 |
|
|
−12 [−19, −4] |
||
|
BID = twice daily. TID = three times daily. |
||
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
BYETTA (exenatide) 250 mcg/mL is a clear, colorless solution supplied as:
- •
- 5 mcg per dose, 60 doses, 1.2 mL prefilled pen, NDC 0310-6512-01
- •
- 10 mcg per dose, 60 doses, 2.4 mL prefilled pen, NDC 0310-6524-01
16.2 Storage and Handling
- •
- Store BYETTA in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- After first use, BYETTA can be kept at a temperature not to exceed 77°F (25°C).
- •
- Do not freeze. Do not use BYETTA if it has been frozen.
- •
- Protect BYETTA from light.
- •
- Discard the pen 30 days after first use, even if some drug remains in the pen.
- •
- Use a puncture-resistant container to discard the needles. Do not reuse or share needles.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Never Share a BYETTA Pen Between Patients
Advise patients that they must never share a BYETTA pen with another person, even if the needle is changed, because doing so carries a risk for transmission of blood-borne pathogens [see Warnings and Precautions (5.1)].
Acute Pancreatitis
Inform patients that persistent severe abdominal pain that may radiate to the back and which may or may not be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Instruct patients to promptly discontinue BYETTA and contact their physician if persistent severe abdominal pain occurs [see Warnings and Precautions (5.2)].
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
Inform patients that the risk of hypoglycemia is increased when BYETTA is used in combination with an agent that induces hypoglycemia, such as a sulfonylurea or insulin. Educate patients on the signs and symptoms of hypoglycemia [see Warnings and Precautions (5.3)].
Acute Kidney Injury
Inform patients treated with BYETTA of the potential risk for worsening renal function and about associated signs and symptoms of renal dysfunction, as well as the possibility of dialysis as a medical intervention if renal failure occurs [see Warnings and Precautions (5.4)].
Drug-Induced Thrombocytopenia
Inform patients that drug-induced immune-mediated thrombocytopenia has been reported during use of exenatide. Inform patients that if symptoms of thrombocytopenia occur, stop taking BYETTA and seek medical advice promptly [see Warnings and Precautions (5.8)].
Hypersensitivity Reactions
Inform patients that serious hypersensitivity reactions have been reported during postmarketing use of BYETTA. If symptoms of hypersensitivity reactions occur, instruct patients to stop taking BYETTA and seek medical advice promptly [see Warnings and Precautions (5.7)].
Acute Gallbladder Disease
Inform patients of the potential risk for cholelithiasis or cholecystitis. Instruct patients to contact their physician if cholelithiasis or cholecystitis is suspected for appropriate clinical follow-up [see Warnings and Precautions (5.9)].
Pregnancy
Advise patients to inform their physicians if they are pregnant or intend to become pregnant.
Instructions
Instruct patients to administer BYETTA as a subcutaneous injection in the thigh, abdomen, or upper arm at any time within the 60-minute period before the morning and evening meals (or before the two main meals of the day, approximately 6 hours or more apart). Do not administer BYETTA after a meal. If a dose is missed, resume the treatment regimen as prescribed with the next scheduled dose.
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
BYETTA® is a registered trademark of the AstraZeneca group of companies.
|
MEDICATION GUIDE BYETTA® (bye-A-tuh) (exenatide) injection, for subcutaneous use |
|||||
|
Read this Medication Guide and the Instructions for Use that comes with BYETTA before you start using it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have questions about BYETTA after reading this information, ask your healthcare provider or pharmacist. |
|||||
|
What is the most important information I should know about BYETTA?
|
|||||
|
What is BYETTA?
|
|||||
|
Who should not use BYETTA? Do not use BYETTA if:
|
|||||
|
|
||||
|
|||||
|
Before taking BYETTA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. BYETTA may affect the way some medicines work and some other medicines may affect the way BYETTA works. Especially tell your healthcare provider if you take:
|
|||||
|
How should I take BYETTA? See the Instructions for Use that comes with BYETTA for instructions for using the BYETTA Pen and injecting BYETTA.
|
|||||
|
What are the possible side effects of BYETTA? BYETTA may cause serious side effects, including:
|
|||||
|
|
|
|
||
|
Talk with your healthcare provider about how to treat low blood sugar.
|
|||||
|
The most common side effects of BYETTA include: |
|||||
|
|
|
|||
|
Nausea is most common when you first start using BYETTA but may decrease over time. Talk to your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of BYETTA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||||
|
How should I store BYETTA?
|
|||||
|
General information about the safe and effective use of BYETTA. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BYETTA for a condition for which it was not prescribed. Do not give BYETTA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about BYETTA that is written for health professionals. |
|||||
|
What are the ingredients in BYETTA? Active ingredient: exenatide Inactive ingredients: metacresol, mannitol, glacial acetic acid, and sodium acetate trihydrate in water for injection. BYETTA® is a registered trademark of the AstraZeneca group of companies. All other trademarks are the trademarks of their respective owners.
Distributed by: AstraZeneca Pharmaceuticals LP Wilmington, DE 19850
For more information, call 1-800-236-9933. |
|||||
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 06/2022
Package/Label Display Panel – 5 mcg
5 mcg per dose
NDC 0310-6512-01
Byetta® exenatide injection
250 mcg/mL, 1.2 mL
Dispense the enclosed Medication Guide to each patient
For Single Patient Use Only
Each prefilled pen will deliver 60 subcutaneous doses, 5 mcg per dose
Rx only
SUBCUTANEOUS USE ONLY
REFRIGERATE – DO NOT FREEZE
DO NOT TRANSFER THIS MEDICATION TO A SYRINGE
Pen needles not included
Ask your healthcare provider which pen needle length and gauge is best for you
Use 29 (thin), 30, or 31 (thinner) gauge disposable pen needles
AstraZeneca
Package/Label Display Panel – 10 mcg
10 mcg per dose
NDC 0310-6524-01
Byetta® exenatide injection
250 mcg/mL, 2.4 mL
Dispense the enclosed Medication Guide to each patient
For Single Patient Use Only
Each prefilled pen will deliver 60 subcutaneous doses, 10 mcg per dose
Rx only
SUBCUTANEOUS USE ONLY
REFRIGERATE – DO NOT FREEZE
DO NOT TRANSFER THIS MEDICATION TO A SYRINGE
Pen needles not included
Ask your healthcare provider which pen needle length and gauge is best for you
Use 29 (thin), 30, or 31 (thinner) gauge disposable pen needles
AstraZeneca


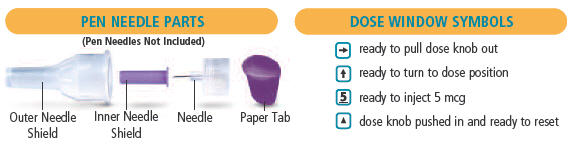






 is in the dose window. If not, turn dose knob away from you (clockwise)
is in the dose window. If not, turn dose knob away from you (clockwise)  is in the dose window.
is in the dose window.  is in the dose window.
is in the dose window. . Make sure that the 5 with the line under it is in the center of the dose window.
. Make sure that the 5 with the line under it is in the center of the dose window. , see
, see 


 is in the center of the dose window
is in the center of the dose window 
 is in the dose window.
is in the dose window.


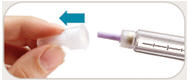
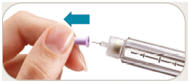



 is in the dose window. If not, turn dose knob away from you (clockwise)
is in the dose window. If not, turn dose knob away from you (clockwise)  is in the dose window.
is in the dose window. is in the dose window.
is in the dose window. . Make sure that the 5 with the line under it is in the center of the dose window.
. Make sure that the 5 with the line under it is in the center of the dose window. , see
, see
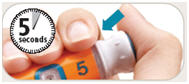

 is in the center of the dose window.
is in the center of the dose window.
 is in the dose window.
is in the dose window.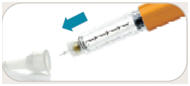


 is in the center of the dose window.
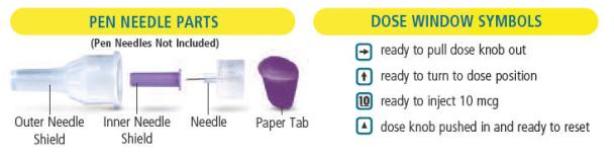
is in the center of the dose window.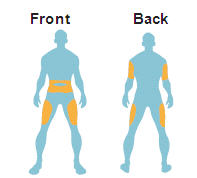

 appears.
appears.


 is in the center of the dose window.
is in the center of the dose window.










 is in the dose window. If not, turn dose knob away from you (clockwise)
is in the dose window. If not, turn dose knob away from you (clockwise)  is in the dose window.
is in the dose window. is in the dose window.
is in the dose window. . Make sure that the 10 with the line under it is in the center of the dose window.
. Make sure that the 10 with the line under it is in the center of the dose window. , see
, see


 is in the center of the dose window
is in the center of the dose window 
 is in the dose window.
is in the dose window.


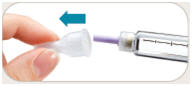
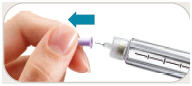



 is in the dose window. If not, turn dose knob away from you (clockwise)
is in the dose window. If not, turn dose knob away from you (clockwise)  is in the dose window.
is in the dose window. is in the dose window.
is in the dose window. . Make sure that the 10 with the line under it is in the center of the dose window.
. Make sure that the 10 with the line under it is in the center of the dose window. , see
, see


 is in the center of the dose window.
is in the center of the dose window.
 is in the dose window.
is in the dose window.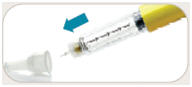
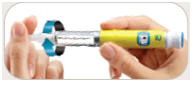

 is in the center of the dose window.
is in the center of the dose window.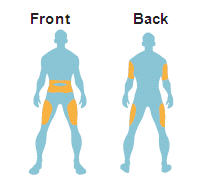

 appears.
appears.


 is in the center of the dose window.
is in the center of the dose window.