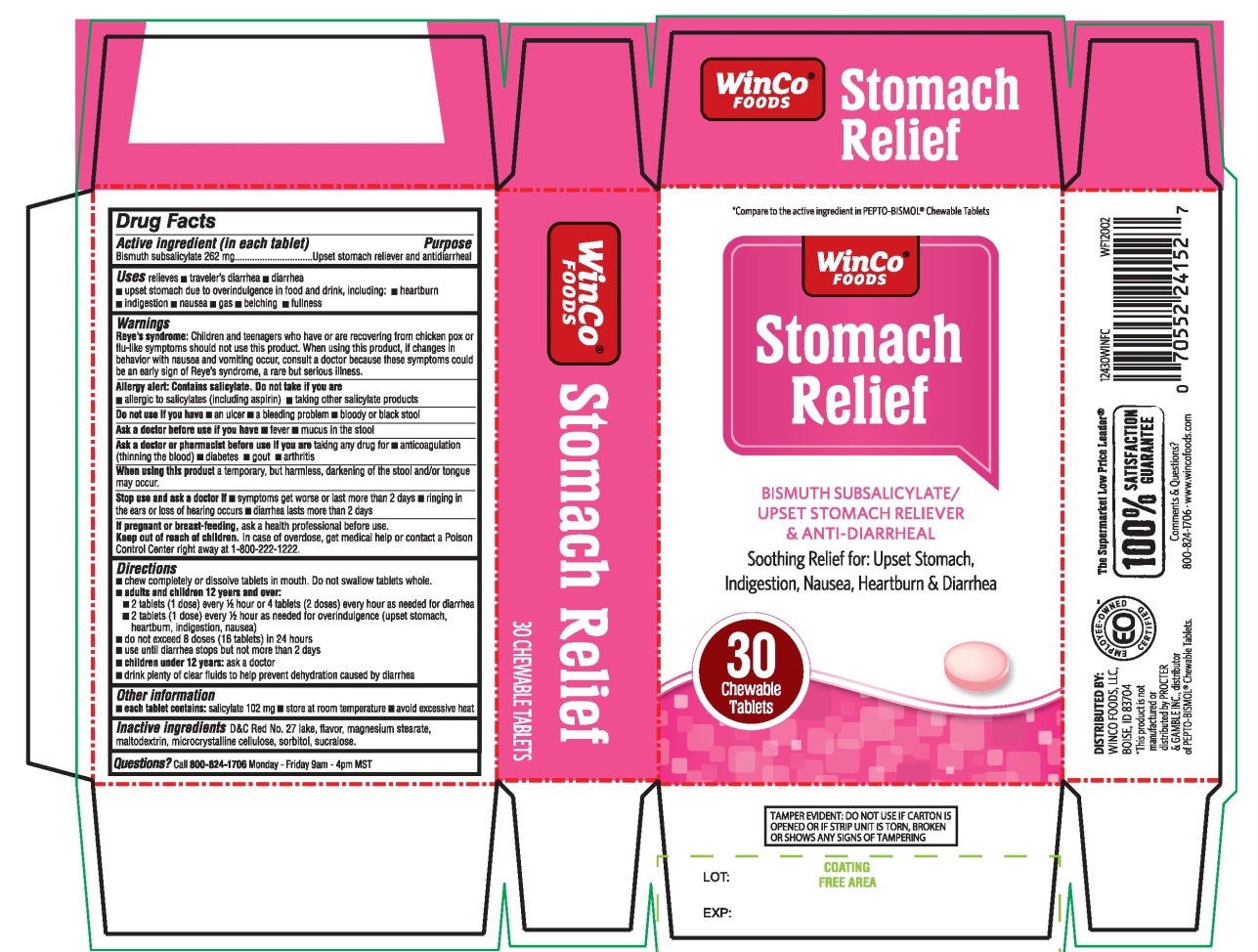
Uses
relieves:
- ▪
- travelers’ diarrhea
- ▪
- diarrhea
- ▪
- upset stomach due to overindulgence in food and drink, including:
- ▪
- heartburn
- ▪
- indigestion
- ▪
- nausea
- ▪
- gas
- ▪
- belching
- ▪
- fullness
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
- ▪
- allergic to salicylates (including aspirin)
- ▪
- taking other salicylate products
Do not use if you have
- ▪
- an ulcer
- ▪
- a bleeding problem
- ▪
- bloody or black stool
Ask a doctor before use if you have
- ▪
- fever
- ▪
- mucus in the stool
Ask a doctor or pharmacist before use if you are taking any drug for
- ▪
- anticoagulation (thinning of the blood)
- ▪
- diabetes
- ▪
- gout
- ▪
- arthritis
Directions
- ▪
- chew completely or dissolve tablets in mouth. Do not swallow tablets whole.
- ▪
- adults and children 12 years and over: 2 tablets (1 dose) every ½ hour or 4 tablets (2 doses) every hour as needed for diarrhea
- ▪
- 2 tablets (1 dose) every ½ hour as needed for overindulgence (upset stomach, heartburn, indigestion, nausea)
- ▪
- do not exceed 8 doses (16 tablets) in 24 hours
- ▪
- use until diarrhea stops but not more than 2 days
- ▪
- children under 12 years: ask a doctor
- ▪
- drink plenty clear fluids to help prevent dehydration caused by diarrhea.
Other information
- ▪
- each tablet contains: salicylate 102 mg
- ▪
- store at room temperature.
- ▪
- avoid excessive heat
- ▪
- keep this carton for future reference on full labeling
TAMPER EVIDENT: Do not use if printed inner wrap is broken or missing.
Inactive ingredients
D&C Red No. 27 lake, flavor, magnesium stearate, maltodextrin, microcrystalline cellulose, sorbitol, sucralose.
Principal Display
Winco® FOODS
NDC# 67091-364-30
Compare to the active ingredient in PEPTO-BISMOL® Chewable Tablets
Stomach Relief
BISMUTH SUBSALICYLATE/
UPSET STOMACH RELIEVER & ANTI-DIARRHEAL
Soothing Relief for: Upset stomach, Indigestion, Nausea, Heartburn, Diarrhea
The Supermarket Low Price Leader®
100% SATISFACTION GUARANTEE
Comments & Question?
800-824-1706 www.wincofoods.com
30 Chewable Tablets
DISTRIBUTED BY:
WINCO FOODS, LLC,
BOISE, ID 83704
*This product is not manufactured or distributed by PROCTER & GAMBLE INC., distributor of PEPTO-BISMOL® Chewable Tablets
If any reason you are not satisfied with this product, please return it to the store where purchased for a full refund.