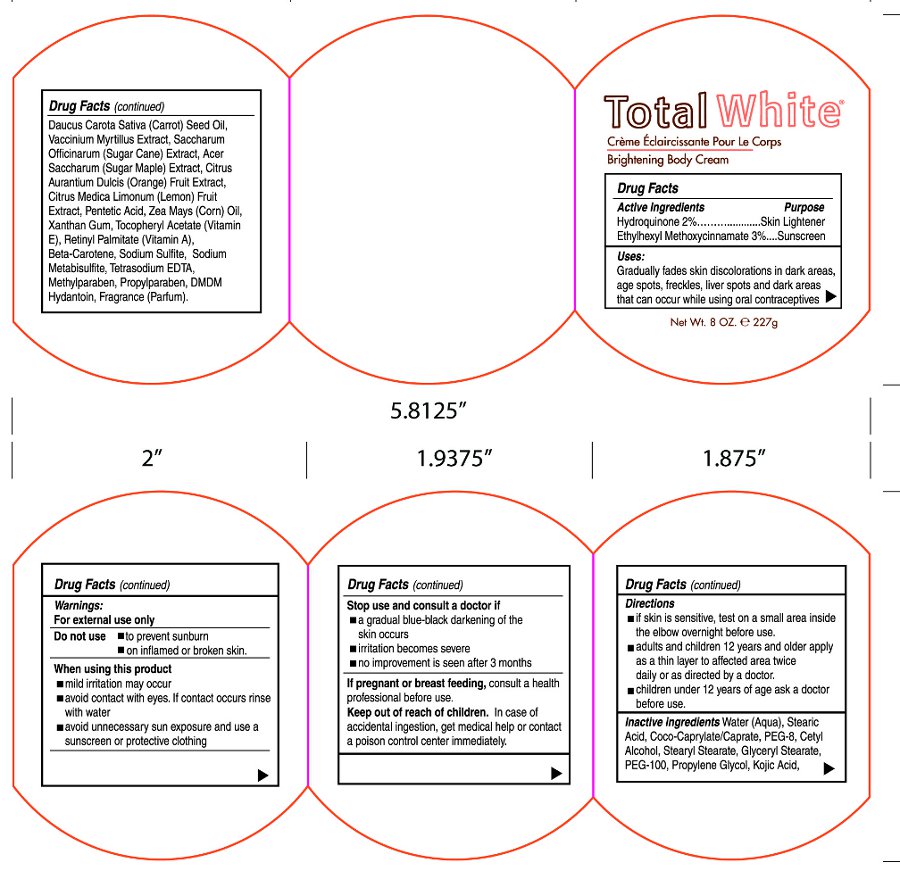
Purpose
Hydroquinone 2% ............................... Skin Lightener
Ethylhexyl Methoxycinnamate 3% ..... Sunscreen
Uses:
Gradually fades skin discolorations in dark areas, age spots, freckles, liver spots and dark areas that can occur while using oral contraceptives
When using this product
• Mild irritation may occur
• Avoid contact with eyes. If contact occurs rinse with water.
• Avoid unnecessary sun exposure and use a sunscreen or protective clothing
Stop use and consult a doctor if
• A gradual blue-black darkening of the skin ocurs
• Irritation becomes severe
• No improvement is seen after 3 months
Keep out of reach of children.
In case of accidental ingestion, get medical help or contact a poison control center immediately.
Directions
• If skin is sensitive, test on a small area inside the elbow overnight before use.
• adults and children 12 years and older apply as a thin layer to affected area twice daily or as directed by a doctor.
• Children under 12 years of age ask a doctor before use.
Inactive ingredients
Water (Aqua), Coco-Caprylate/Caprate, Stearic Acid, PEG-8, Cetyl Alcohol, Stearyl Stearate, Glyceryl Stearate, PEG-100, Propylene Glycol, Kojic Acid, Daucus Carota Sative (Carrot) Seed Oil, Vaccinium Myrtillus Extract, Saccharum Officinarum (Sugar Cane) Extract, Acer Saccharum (Sugar Maple) Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Citrus Medica Limonum (Lemon) Fruit Extract, Pentetic Acid, Zea Mays (Corn) Oil, Xanthan Gum, Tocopheryl Acetate (Vitamin E), Retinyl Palmitate (Vitamin A), Beta-Carotene, Sodium Sulfite, Sodium Metabisulfite, Tetrasodium EDTA, Methylparaben, Propylparaben, DMDM Hydantoin, Fragrance (Parfum)
PRINCIPAL DISPLAY PANEL
Total White
Brightening Body Cream
Lightens Skin
Natural Bio-vegetal Activation
With Carrot Oil
8 OZ e 227 g Distributed by Heaven Scent, Miami FL
shimonb@yzyinc.com 877-999-3534
Tottenham, London N17 OQT, UK
MADE IN USA

Total White
Brightening Body Cream
Total White Brightening Cream diminishes the appearance of dark spots and skin discolorations caused by acne, aging, sun damage and pregnancey. This rich cream is formulated with Carrot Oil and Vitamins A and E; it effectivly moisturizes and nourishes your skin, while it protects from future damage. It safely brightens and evens dark spots for younger, healthier, more radiant looking skin.

Total White Brightening Body Cream diminishes the appearance of dark spots and skin discolorations caused by acne, aging, sun damage, and pregnancy. This rich cream is formulated with Carrot Oil and Vitamins A and E, it effectively moisturizes and nourishes your skin, while it protects against future damage. It safely brightens and evens dark spots for younger, healthier more radiant looking skin.

Total White Brightening Body Cream With carrot oil Lightens SkinNatural Bio-Vegetal Activator
