PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Aleor Dermaceuticals Ltd.
Clobetasol Propionate Ointment USP, 0.05%
Rx only
FOR DERMATOLOGIC USE ONLY
NOT FOR OPHTHALMIC USE.
NET WT 15 grams
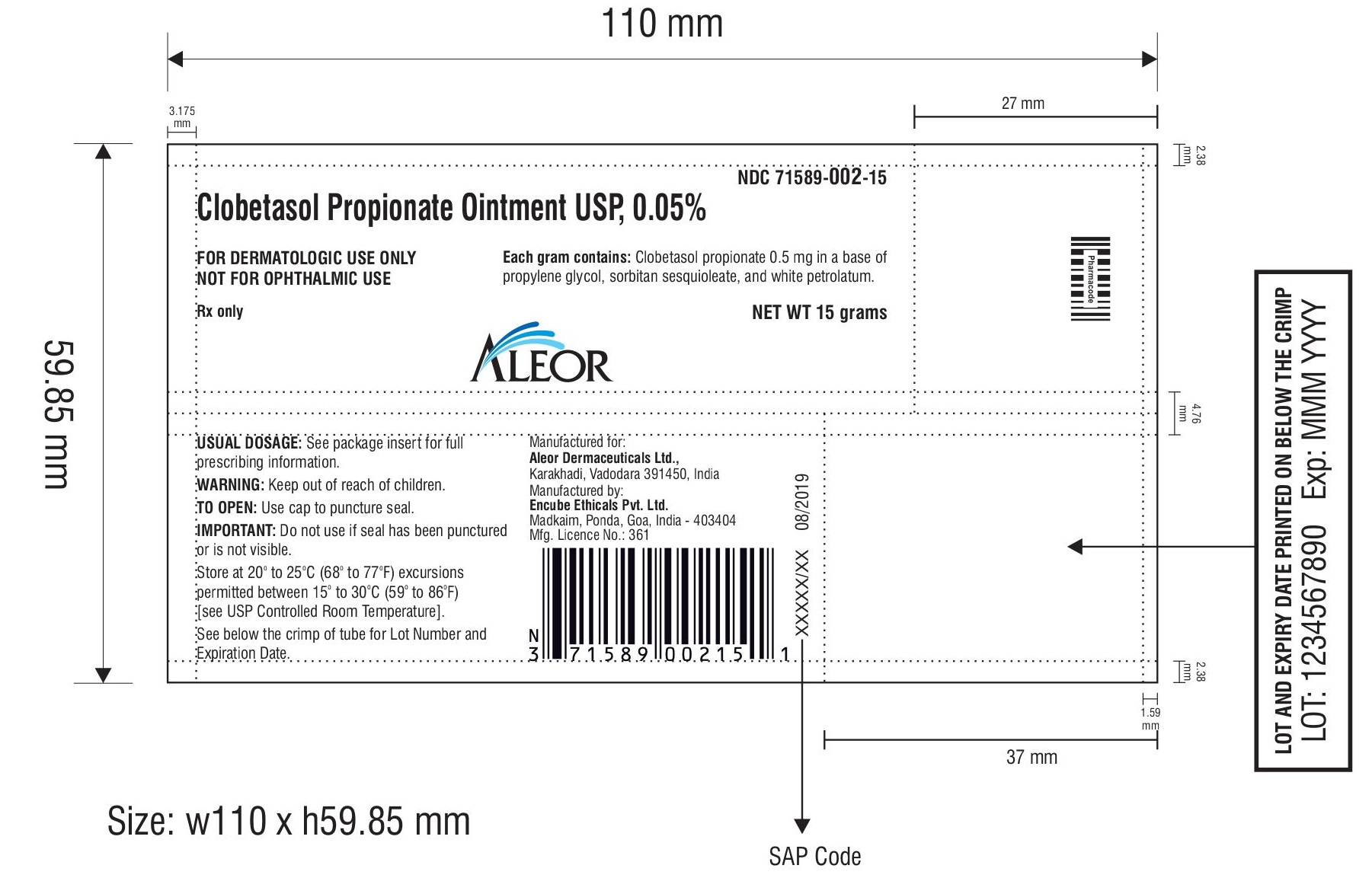
Aleor Dermaceuticals Ltd.
Clobetasol Propionate Ointment USP, 0.05%
Rx only
FOR DERMATOLOGIC USE ONLY
NOT FOR OPHTHALMIC USE.
NET WT 15 grams
