AMINOSYN II- isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, tryptophan, valine, alanine, arginine, aspartic acid, glutamic acid, histidine, proline, serine, n-acetyl-tyrosine, and glycine injection, solution
Hospira, Inc.
----------
Aminosyn® II
Sulfite-Free
DESCRIPTION
Aminosyn® II, Sulfite-Free, (an amino acid injection) is a sterile, nonpyrogenic solution for intravenous infusion. Aminosyn II is oxygen sensitive. The following formulations are available:
| Aminosyn II Formulations
Essential Amino Acids (mg/100 mL) |
|||
|---|---|---|---|
| Aminosyn II
| 7%
| 8.5%
| 10%
|
|
Isoleucine |
462 |
561 |
660 |
|
Leucine |
700 |
850 |
1000 |
|
Lysine (acetate)* |
735 |
893 |
1050 |
|
Methionine |
120 |
146 |
172 |
|
Phenylalanine |
209 |
253 |
298 |
|
Threonine |
280 |
340 |
400 |
|
Tryptophan |
140 |
170 |
200 |
|
Valine |
350 |
425 |
500 |
|
*Amount cited is for lysine alone and does not include the acetate. |
|||
| Nonessential Amino Acids (mg/100 mL)
|
|||
|---|---|---|---|
| Aminosyn II
| 7%
| 8.5%
| 10%
|
|
Alanine |
695 |
844 |
993 |
|
Arginine |
713 |
865 |
1018 |
|
L-Aspartic Acid |
490 |
595 |
700 |
|
L-Glutamic Acid |
517 |
627 |
738 |
|
Histidine |
210 |
255 |
300 |
|
Proline |
505 |
614 |
722 |
|
Serine |
371 |
450 |
530 |
|
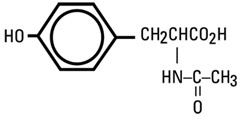
N-AcetyI-L-Tyrosine |
189 |
230 |
270 |
|
Glycine |
350 |
425 |
500 |
| Product Characteristics
|
|||
|---|---|---|---|
| Aminosyn II
| 7%
| 8.5%
| 10%
|
|
Protein Equivalent (approx. grams/liter) |
70 |
85 |
100 |
|
Total Nitrogen (grams/liter) |
10.7 |
13.0 |
15.3 |
|
Osmolarity (mOsmol/liter) |
589 |
706 |
1040 |
|
pHa (range) |
5.8 5.0—6.5 |
5.8 5.0—6.5 |
5.8 5.0—6.5 |
|
a Contains sodium hydroxide for pH adjustment. |
|||
| Electrolytes (mEq/Liter)
|
|||
|---|---|---|---|
| Aminosyn II
| 7%
| 8.5%
| 10%
|
|
Sodium (Na+)b |
25 |
32 |
38 |
|
Acetate(C2H3O2−)c |
50 |
61 |
72 |
|
b Na+ from pH adjustor. c Includes acetate from lysine acetate. |
|||
The formulas for the individual amino acids present in Aminosyn® II, are as follows:
| Essential Amino Acids
|
|
|---|---|
|
Isoleucine, USP |
C6H13NO2 |
|
Leucine, USP |
C6H13NO2 |
|
Lysine Acetate, USP |
C6H14N2O2 • CH3COOH |
|
Methionine, USP |
C5H11NO2S |
|
Phenylalanine, USP |
C9H11NO2 |
|
Threonine, USP |
C4H9NO3 |
|
Tryptophan, USP |
C11H12N2O2 |
|
Valine, USP |
C5H11NO2 |
| Nonessential Amino Acids
|
|
|---|---|
|
Alanine, USP |
C3H7NO2 |
|
Arginine, USP |
C6H14N4O2 |
|
L-Aspartic Acid |
C4H7NO4 |
|
HO2CCH2CH(NH2)CO2H |
|
|
L-Glutamic Acid |
C5H9NO4 |
|
HO2CCH2CH2CH(NH2)CO2H |
|
|
Glycine, USP |
C2H5NO2 |
|
Histidine, USP |
C6H9N3O2 |
|
Proline, USP |
C5H9NO2 |
|
Serine, USP |
C3H7NO3 |
|
N-Acetyl-L-Tyrosine |
C11H13NO4 |
|
|
|
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly.
Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials.
Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
CLINICAL PHARMACOLOGY
Aminosyn II, Sulfite-Free, (an amino acid injection) provides crystalline amino acids to promote protein synthesis and wound healing, and to reduce the rate of endogenous protein catabolism. Aminosyn II, given by central venous infusion in combination with concentrated dextrose, electrolytes, vitamins, trace metals, and ancillary fat supplements, constitutes total parenteral nutrition (TPN). Aminosyn II can also be administered by peripheral vein with dextrose and maintenance electrolytes. Intravenous fat emulsion may be substituted for part of the carbohydrate calories during either TPN or peripheral vein administration of Aminosyn II.
INDICATIONS AND USAGE
Aminosyn II, Sulfite-Free, (an amino acid injection) infused with dextrose by peripheral vein infusion is indicated as a source of nitrogen in the nutritional support of patients with adequate stores of body fat, in whom, for short periods of time, oral nutrition cannot be tolerated, is undesirable, or inadequate.
SUPPLEMENTAL ELECTROLYTES, IN ACCORDANCE WITH THE PRESCRIPTION OF THE ATTENDING PHYSICIAN, MUST BE ADDED TO AMINOSYN II SOLUTIONS WITHOUT ELECTROLYTES.
Aminosyn II can be administered peripherally with dilute (5 to 10%) dextrose solution and I.V. fat emulsion as a source of nutritional support. This form of nutritional support can help to preserve protein and reduce catabolism in stress conditions where oral intake is inadequate.
When administered with concentrated dextrose solution with or without fat emulsions, Aminosyn II is also indicated for central vein infusion to prevent or reverse negative nitrogen balance in patients where: (a) the alimentary tract, by the oral, gastrostomy or jejunostomy route cannot or should not be used; (b) gastrointestinal absorption of protein is impaired; (c) metabolic requirements for protein are substantially increased as with extensive burns and (d) morbidity and mortality may be reduced by replacing amino acids lost from tissue breakdown, thereby preserving tissue reserves, as in acute renal failure.
CONTRAINDICATIONS
This preparation should not be used in patients with hepatic coma or metabolic disorders involving impaired nitrogen utilization.
WARNINGS
Intravenous infusion of amino acids may induce a rise in blood urea nitrogen (BUN), especially in patients with impaired hepatic or renal function. Appropriate laboratory tests should be performed periodically and infusion discontinued if BUN levels exceed normal postprandial limits and continue to rise. It should be noted that a modest rise in BUN normally occurs as a result of increased protein intake.
Administration of amino acid solutions to a patient with hepatic insufficiency may result in serum amino acid imbalances, metabolic alkalosis, prerenal azotemia, hyperammonemia, stupor and coma.
Administration of amino acid solutions in the presence of impaired renal function may augment an increasing BUN, as does any protein dietary component.
Solutions containing sodium ion should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
Solutions which contain potassium ion should be used with great care, if at all, in patients with hyperkalemia, severe renal failure and in conditions in which potassium retention is present.
Solutions containing acetate ion should be used with great care in patients with metabolic or respiratory alkalosis. Acetate should be administered with great care in those conditions in which there is an increased level or an impaired utilization of this ion, such as severe hepatic insufficiency.
Hyperammonemia is of special significance in infants, as it can result in mental retardation. Therefore, it is essential that blood ammonia levels be measured frequently in infants.
Instances of asymptomatic hyperammonemia have been reported in patients without overt liver dysfunction. The mechanisms of this reaction are not clearly defined, but may involve genetic defects and immature or subclinically impaired liver function.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
PRECAUTIONS
Special care must be taken when administering glucose to provide calories in diabetic or prediabetic patients.
Feeding regimens which include amino acids should be used with caution in patients with history of renal disease, pulmonary disease, or with cardiac insufficiency so as to avoid excessive fluid accumulation.
The effect of infusion of amino acids, without dextrose, upon carbohydrate metabolism of children is not known at this time.
Nitrogen intake should be carefully monitored in patients with impaired renal function.
For long-term total nutrition, or if a patient has inadequate fat stores, it is essential to provide adequate exogenous calories concurrently with the amino acids. Concentrated dextrose solutions are an effective source of such calories. Such strongly hypertonic nutrient solutions should be administered through an indwelling intravenous catheter with the tip located in the superior vena cava.
SPECIAL PRECAUTIONS FOR CENTRAL VENOUS INFUSIONS
ADMINISTRATION BY CENTRAL VENOUS CATHETER SHOULD BE USED ONLY BY THOSE FAMILIAR WITH THIS TECHNIQUE AND ITS COMPLICATIONS.
Central vein infusion (with added concentrated carbohydrate solutions) of amino acid solutions requires a knowledge of nutrition as well as clinical expertise in recognition and treatment of complications. Attention must be given to solution preparation, administration and patient monitoring. IT IS ESSENTIAL THAT A CAREFULLY PREPARED PROTOCOL BASED ON CURRENT MEDICAL PRACTICES BE FOLLOWED, PREFERABLY BY AN EXPERIENCED TEAM.
SUMMARY HIGHLIGHTS OF COMPLICATIONS (consult current medical literature).
-
Technical
The placement of a central venous catheter should be regarded as a surgical procedure. One should be fully acquainted with various techniques of catheter insertion. For details of technique and placement sites, consult the medical literature. X-ray is the best means of verifying catheter placement. Complications known to occur from the placement of central venous catheters are pneumothorax, hemothorax, hydrothorax, artery puncture and transection, injury to the brachial plexus, malposition of the catheter, formation of arteriovenous fistula, phlebitis, thrombosis and air and catheter emboli.
-
Septic
The constant risk of sepsis is present during administration of total parenteral nutrition. It is imperative that the preparation of the solution and the placement and care of catheters be accomplished under strict aseptic conditions.
Solutions should ideally be prepared in the hospital pharmacy in a laminar flow hood using careful aseptic technique to avoid inadvertent touch contamination. Solutions should be used promptly after mixing. Storage should be under refrigeration and limited to a brief period of time, preferably less than 24 hours.
Administration time for a single container and set should never exceed 24 hours.
-
Metabolic
The following metabolic complications have been reported with TPN administration: metabolic acidosis and alkalosis, hypophosphatemia, hypocalcemia, osteoporosis, glycosuria, hyperglycemia, hyperosmolar nonketotic states and dehydration, rebound hypoglycemia, osmotic diuresis and dehydration, elevated liver enzymes, hypo- and hypervitaminosis, electrolyte imbalances and hyperammonemia in children. Frequent evaluations are necessary especially during the first few days of therapy to prevent or minimize these complications.
Administration of glucose at a rate exceeding the patient's utilization rate may lead to hyperglycemia, coma and death.
Pregnancy Category C
Animal reproduction studies have not been conducted with Aminosyn II, Sulfite-Free, (an amino acid injection). It is not known whether Aminosyn II can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Aminosyn II should be given to a pregnant woman only if clearly needed.
Geriatric Use
Clinical studies of Aminosyn II have not been performed to determine whether patients over 65 years respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal functions.
CLINICAL EVALUATION AND LABORATORY DETERMINATIONS, AT THE DISCRETION OF THE ATTENDING PHYSICIAN, ARE NECESSARY FOR PROPER MONITORING DURING ADMINISTRATION. Do not withdraw venous blood for blood chemistries through the peripheral infusion site, as interference with estimations of nitrogen containing substances may occur. Blood studies should include glucose, urea nitrogen, serum electrolytes, ammonia, cholesterol, acid-base balance, serum proteins, kidney and liver function tests, osmolarity and hemogram. White blood count and blood cultures are to be determined if indicated. Urinary osmolality and glucose should be determined as necessary.
Aminosyn II contains no more than 25 mcg/L of aluminum.
Drug Interactions
Because of its antianabolic activity, concurrent administration of tetracycline may reduce the potential anabolic effects of amino acids infused with dextrose as part of a parenteral feeding regimen.
Additives may be incompatible. Consult with pharmacist if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
ADVERSE REACTIONS
Peripheral Infusions
A 3.5% to 5% solution of amino acids (without additives) is slightly hypertonic. Local reactions consisting of a warm sensation, erythema, phlebitis and thrombosis at the infusion site have occurred with peripheral intravenous infusion of amino acids particularly if other substances, such as antibiotics, are also administered through the same site. In such cases the infusion site should be changed promptly to another vein. Use of large peripheral veins, inline filters, and slowing the rate of infusion may reduce the incidence of local venous irritation. Electrolyte additives should be spread throughout the day. Irritating additive medications may need to be infused at another venous site.
Generalized flushing, fever and nausea also have been reported during peripheral infusions of amino acid solutions.
OVERDOSAGE
In the event of overhydration or solute overload, re-evaluate the patient and institute appropriate corrective measures. See WARNINGS and PRECAUTIONS.
DOSAGE AND ADMINISTRATION
The total daily dose of the solution depends on the daily protein requirements and on the patient's metabolic and clinical response. In many patients, provision of adequate calories in the form of hypertonic dextrose may require the administration of exogenous insulin to prevent hyperglycemia and glycosuria. To prevent rebound hypoglycemia, a solution containing 5% dextrose should be administered when hypertonic dextrose infusions are abruptly discontinued.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. COLOR VARIATION FROM PALE YELLOW TO YELLOW IS NORMAL AND DOES NOT ALTER EFFICACY.
-
Peripheral Vein Nutritional Maintenance
Aminosyn II 3.5%, Sulfite-Free, (an amino acid injection) together with dextrose in concentrations of 5% to 10% is suitable for administration by peripheral vein. This solution is not intended for central vein infusion since it does not contain adequate amounts of amino acids or electrolytes for administration with high concentrations of dextrose. Aminosyn II 7%, 8.5% or 10% may be diluted with sterile water for injection or 5 to 10% Dextrose Injection to achieve a final amino acid concentration of 3.5, 4.25 or 5% for peripheral administration.
For peripheral intravenous infusion, 1 to 1.5 g/kg/day of total amino acids will reduce protein catabolism. Infusion or ingestion of carbohydrate or lipid will not reduce the nitrogen sparing effect of intravenous amino acid infusions at this dose.
As with all intravenous fluid therapy, the primary aim is to provide sufficient water to compensate for insensible, urinary and other (nasogastric suction, fistula drainage, diarrhea) fluid losses. Total fluid requirements, as well as electrolyte and acid-base needs, should be estimated and appropriately administered.
For an amino acid solution of specified total concentration, the volume required to meet amino acid requirements per 24 hours can be calculated. After making an estimate of the total daily fluid (water) requirement, the balance of fluid needed beyond the volume of amino acid solution required can be provided either as a noncarbohydrate or a carbohydrate-containing electrolyte solution. I.V. lipid emulsion may be substituted for part of the carbohydrate-containing solution. Vitamins and additional electrolytes as needed for maintenance or to correct imbalances may be added to the amino acid solution.
If desired, only one-half of an estimated daily amino acid requirement of 1.5 g/kg can be given on the first day. Amino acids together with dextrose in concentrations of 5% to 10% infused into a peripheral vein can be continued while oral nutrition is impaired. However, if a patient is unable to take oral nourishment for a prolonged period of time, institution of total parenteral nutrition with exogenous calories should be considered.
-
Central Vein Total Parenteral Nutrition
For central vein infusion with concentrated dextrose solution, alone or with I.V. lipid, the total daily dose of the amino acid solution depends upon daily protein requirements and the patient's metabolic and clinical response. The determination of nitrogen balance and accurate daily body weights, corrected for fluid balance, are probably the best means of assessing individual protein requirements.
Adults
Solutions containing 3.5 to 5% amino acids with 5 to 10% glucose may be infused with a fat emulsion by peripheral vein to provide approximately 1400 to 2000 kcal/day. Fat emulsion administration should be considered when prolonged parenteral nutrition is required in order to prevent essential fatty acid deficiency (E.F.A.D.). Serum lipids should be monitored for evidence of E.F.A.D. in patients maintained on fat-free TPN.
Aminosyn II 7%, 8.5% and 10% solutions should only be infused via a central vein when admixed with sufficient dextrose to provide full caloric requirements in patients who require prolonged total parenteral nutrition. I.V. lipid may be administered to provide part of the calories, if desired. Serum lipids should be monitored for evidence of essential fatty acid deficiency in patients maintained on fat-free TPN.
Total parenteral nutrition (TPN) may be started with 10% dextrose added to the calculated daily requirement of amino acids (1.5 g/kg for a metabolically stable patient). Dextrose content is gradually increased over the next few days to the estimated daily caloric need as the patient adapts to the increasing amounts of dextrose. Each gram of dextrose provides approximately 3.4 kcal. Each gram of fat provides 9 kcal.
The average depleted major surgical patient with complications requires between 2500 and 4000 kcal and between 12 and 24 grams of nitrogen per day. An adult patient in an acceptable weight range with restricted activity who is not hypermetabolic, requires about 30 kcal/kg of body weight/day. Average daily adult fluid requirements are between 2500 and 3000 mL and may be much higher with losses from fistula drainage or severe burns. Typically, a hospitalized patient may lose 12 to 18 grams of nitrogen a day, and in severe trauma the daily loss may be 20 to 25 grams or more.
Aminosyn II solutions without electrolytes are intended for patients requiring individualized electrolyte therapy. Sodium, chloride, potassium, phosphate, calcium and magnesium are major electrolytes which should be added to Aminosyn II as required.
SERUM ELECTROLYTES SHOULD BE MONITORED AS INDICATED. Electrolytes may be added to the nutrient solution as indicated by the patient's clinical condition and laboratory determinations of plasma values. Major electrolytes are sodium, chloride, potassium, phosphate, magnesium and calcium. Vitamins, including folic acid and vitamin K are required additives. The trace element supplements should be given when long-term parenteral nutrition is undertaken.
Calcium and phosphorus are added to the solution as indicated. The usual dose of phosphorus added to a liter of TPN solution (containing 25% dextrose) is 12 mM. This requirement is related to the carbohydrate calories delivered. Iron is added to the solution or given intramuscularly in depot form as indicated. Vitamin B12, vitamin K and folic acid are given intramuscularly or added to the solution as desired.
Calcium and phosphorus additives are potentially incompatible when added to the TPN admixture. However, if one additive is added to the amino acid container and the other to the container of concentrated dextrose, and if the contents of both containers are swirled before they are combined, then the likelihood of physical incompatibility is reduced.
In patients with hyperchloremic or other metabolic acidosis, sodium and potassium may be added as the acetate or lactate salts to provide bicarbonate alternates.
In adults, hypertonic mixtures of amino acids and dextrose may be safely administered by continuous infusion through a central venous catheter with the tip located in the vena cava. Typically, the 7%, 8.5% or 10% solution is used in equal volume with 50% dextrose to provide an admixture containing 3.5%, 4.25% or 5% amino acids and 25% dextrose respectively.
The rate of intravenous infusion initially should be 2 mL/min and may be increased gradually. If administration should fall behind schedule, no attempt to "catch up" to planned intake should be made. In addition to meeting protein needs, the rate of administration is governed by the patient's glucose tolerance estimated by glucose levels in blood and urine.
Aminosyn II 10% solution, when mixed with an appropriate volume of concentrated dextrose, offers a higher concentration of calories and nitrogen per unit volume. This solution is indicated for patients requiring larger amounts of nitrogen than could otherwise be provided or where total fluid load must be kept to a minimum, for example, patients with renal failure.
Provision of adequate calories in the form of hypertonic dextrose may require exogenous insulin to prevent hyperglycemia and glycosuria. To prevent rebound hypoglycemia, do not abruptly discontinue administration of nutritional solutions.
Pediatric
Pediatric requirements for parenteral nutrition are constrained by the greater relative fluid requirements of the infant and greater caloric requirements per kilogram. Amino acids are probably best administered in a 2.5% concentration. For most pediatric patients on intravenous nutrition, 2.5 grams amino acids/kg/day with dextrose alone or with I.V. lipid calories of 100 to 130 kcal/kg/day is recommended. In cases of malnutrition or stress, these requirements may be increased. It is acceptable in pediatrics to start with a nutritional solution of half strength at a rate of about 60 to 70 mL/kg/day. Within 24 to 48 hours the volume and concentration of the solution can be increased until the full strength pediatric solution (amino acids and dextrose) is given at a rate of 125 to 150 mL/kg/day.
Supplemental electrolytes and vitamin additives should be administered as deemed necessary by careful monitoring of blood chemistries and nutritional status. Addition of iron is more critical in the infant than the adult because of the increasing red cell mass required for the growing infant. Serum lipids should be monitored for evidence of essential fatty acid deficiency in patients maintained on fat-free TPN. Bicarbonate should not be administered during infusion of the nutritional solution unless deemed absolutely necessary.
To ensure the precise delivery of the small volumes of fluid necessary for total parenteral nutrition in infants, accurately calibrated and reliable infusion systems should be used.
A basic solution for pediatric use should contain 25 grams of amino acids and 200 to 250 grams of glucose per 1000 mL, administered from containers containing 250 or 500 mL. Such a solution given at the rate of 145 mL/kg/day provides 130 kcal/kg/day.
WARNING: Do not use flexible container in series connections.
HOW SUPPLIED
| NDC No.
| Concentration
| Container (mL)
|
|---|---|---|
|
0409-4160-03 |
Aminosyn II 7%, Sulfite-Free, (an amino acid injection) |
500 |
|
0409-4162-05 0409-4162-03 |
Aminosyn II 8.5%, Sulfite-Free, (an amino acid injection) |
1000 500 |
|
0409-4164-05 0409-4164-03 |
Aminosyn II 10%, Sulfite-Free, (an amino acid injection) |
1000 500 |
Protect from freezing. Store at 20 to 25ºC (68 to 77ºF). [See USP Controlled Room Temperature.] Avoid exposure to light.
Revised: April, 2008
Printed in USA
EN-1783
Hospira, Inc., Lake Forest, IL 60045 USA
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label - NDC 0409-4160
500 mL
NDC 0409-4160-03
Aminosyn® II 7%
Sulfite-Free
AN AMINO ACID INJECTION
EACH 100 mL CONTAINS: TOTAL AMINO ACIDS APPROX. 7 g.
CONTAINS SODIUM HYDROXIDE FOR pH ADJUSTMENT. ESSENTIAL
AMINO ACIDS/100 mL: ISOLEUCINE 462 mg; LEUCINE 700 mg; LYSINE
(AS ACETATE SALT) 735 mg; METHIONINE 120 mg; PHENYLALANINE
209 mg; THREONINE 280 mg; TRYPTOPHAN 140 mg; VALINE 350 mg.
NONESSENTIAL AMINO ACIDS/100 mL: N-ACETYL-L-TYROSINE
189 mg; ALANINE 695 mg; ARGININE 713 mg; GLYCINE 350 mg;
PROLINE 505 mg; HISTIDINE 210 mg; SERINE 371 mg; L-ASPARTIC
ACID 490 mg; L-GLUTAMIC ACID 517 mg. ELECTROLYTES (mEq/LITER):
SODIUM 25 (FROM pH ADJUSTMENT); ACETATE 50.
pH 5.8 (5.0 TO 6.5)
589 mOsmol/LITER
SPECIFIC GRAVITY = 1.02
ADDITIVES MAY BE INCOMPATIBLE. CONSULT WITH
PHARMACIST, IF AVAILABLE. WHEN INTRODUCING ADDITIVES,
USE ASEPTIC TECHNIQUE, MIX THOROUGHLY AND DO NOT STORE.
SINGLE DOSE CONTAINER. CONTAINS NO BACTERIOSTAT. DISCARD
UNUSED PORTION. FOR I.V. USE. USUAL DOSAGE: SEE INSERT. STERILE,
NONPYROGENIC. STORE AT 20 TO 25°C (68 TO 77°F). [SEE USP CONTROLLED ROOM
TEMPERATURE.] AVOID EXCESSIVE HEAT. PROTECT FROM FREEZING. AVOID
EXPOSURE TO LIGHT. USE ONLY IF SOLUTION
IS CLEAR AND CONTAINER IS UNDAMAGED.
MUST NOT BE USED IN SERIES CONNECTIONS.
3
v
CONTAINS DEHP
RX ONLY
PRINTED IN USA
©HOSPIRA 2004
IM-0634 (9/04)
Hospira
HOSPIRA, INC., LAKE FOREST, IL 60045 USA

PRINCIPAL DISPLAY PANEL - 500 mL Bag Label - NDC 0409-4162
500 mL
NDC 0409-4162-03
Aminosyn® II 8.5%
Sulfite-Free
AN AMINO ACID INJECTION
EACH 100 mL CONTAINS: TOTAL AMINO ACIDS APPROX. 8.5 g. CONTAINS
SODIUM HYDROXIDE FOR pH ADJUSTMENT. ESSENTIAL AMINO
ACIDS/100 mL: ISOLEUCINE 561 mg; LEUCINE 850 mg; LYSINE (AS ACETATE
SALT) 893 mg; METHIONINE 146 mg; PHENYLALANINE 253 mg; THREONINE
340 mg; TRYPTOPHAN 170 mg; VALINE 425 mg. NONESSENTIAL AMINO ACIDS/
100 mL: N-ACETYL-L-TYROSINE 230 mg; ALANINE 844 mg; ARGININE 865 mg;
GLYCINE 425 mg; PROLINE 614 mg; HISTIDINE 255 mg; SERINE 450 mg;
L-ASPARTIC ACID 595 mg; L-GLUTAMIC ACID 627 mg. ELECTROLYTES
(mEq/LITER): SODIUM 32 (FROM pH ADJUSTMENT); ACETATE 61.
pH 5.8 (5.0 to 6.5)
706 mOsmol/LITER
SPECIFIC GRAVITY = 1.03
ADDITIVES MAY BE INCOMPATIBLE. CONSULT WITH PHARMACIST,
IF AVAILABLE. WHEN INTRODUCING ADDITIVES, USE ASEPTIC
TECHNIQUE, MIX THOROUGHLY AND DO NOT STORE.
SINGLE DOSE CONTAINER. CONTAINS NO BACTERIOSTAT. DISCARD UNUSED PORTION. FOR
I.V. USE. USUAL DOSAGE: SEE INSERT. STERILE, NONPYROGENIC. STORE AT 20 TO 25°C
(68 TO 77°F). [SEE USP CONTROLLED ROOM TEMPERATURE.] AVOID EXCESSIVE HEAT.
PROTECT FROM FREEZING. AVOID EXPOSURE TO LIGHT. USE ONLY IF SOLUTION IS CLEAR
AND CONTAINER IS UNDAMAGED. MUST NOT BE
USED IN SERIES CONNECTIONS.
RX ONLY
3
V
CONTAINS DEHP
Hospira
©HOSPIRA 2004
IM-0636 (9/04)
PRINTED IN USA
HOSPIRA, INC., LAKE FOREST, IL 60045 USA

PRINCIPAL DISPLAY PANEL - 500 mL Bag Overwrap - NDC 0409-4162
TO OPEN – TEAR AT NOTCH
500 mL
NDC 0409-4162-03
Aminosyn® II
8.5%
Sulfite-Free
AN AMINO ACID INJECTION
Each 100 mL contains: Total amino acids approx. 8.5 g. Contains sodium hydroxide for
pH adjustment. Essential Amino Acids/100 mL: Isoleucine 561 mg; leucine 850 mg; lysine
(as acetate salt) 893 mg; methionine 146 mg; phenylalanine 253 mg; threonine 340 mg;
tryptophan 170 mg; valine 425 mg. Nonessential Amino Acids/100 mL:
N-acetyl-L-tyrosine 230 mg; alanine 844 mg; arginine 865 mg; glycine 425 mg; proline
614 mg; histidine 255 mg; serine 450 mg; L-aspartic acid 595 mg; L-glutamic acid
627 mg. Electrolytes (mEq/Liter): Sodium 32 (from pH adjustment); acetate 61.
706 mOsmol/Liter
pH 5.8 (5.0 to 6.5)
Specific Gravity = 1.03
Single dose container. The overwrap is a moisture and oxygen barrier. Do not remove unit
from overwrap until ready for use. Visually inspect overwrap for tears or holes. Discard
unit if overwrap is damaged. Use unit promptly when overwrap is opened. Store at 20 to
25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing. After
removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are
found, discard solution as sterility may be impaired.
COLOR VARIATION FROM PALE YELLOW TO YELLOW IS NORMAL AND DOES NOT ALTER
EFFICACY
Rx only
7
OTHER
F WR-0290 (5/08)
Printed in USA
Hospira, Inc., Lake Forest, IL 60045 USA
Hospira

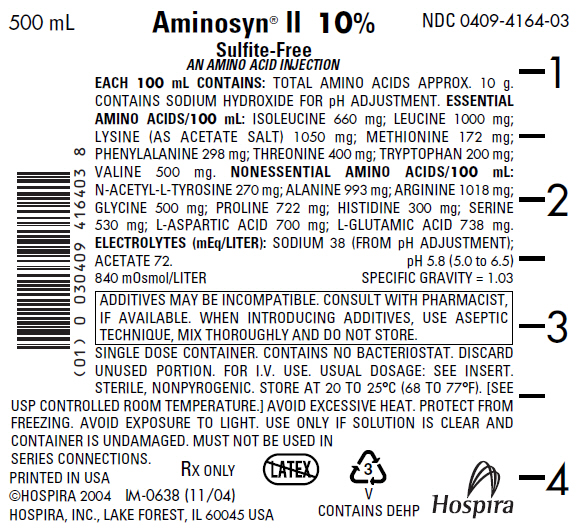
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label - NDC 0409-4164
500 mL
NDC 0409-4164-03
Aminosyn® II 10%
Sulfite-Free
AN AMINO ACID INJECTION
EACH 100 mL CONTAINS: TOTAL AMINO ACIDS APPROX. 10 g.
CONTAINS SODIUM HYDROXIDE FOR pH ADJUSTMENT. ESSENTIAL
AMINO ACIDS/100 mL: ISOLEUCINE 660 mg; LEUCINE 1000 mg;
LYSINE (AS ACETATE SALT) 1050 mg; METHIONINE 172 mg;
PHENYLALANINE 298 mg; THREONINE 400 mg; TRYPTOPHAN 200 mg;
VALINE 500 mg. NONESSENTIAL AMINO ACIDS/100 mL:
N-ACETYL-L-TYROSINE 270 mg; ALANINE 993 mg; ARGININE 1018 mg;
GLYCINE 500 mg; PROLINE 722 mg; HISTIDINE 300 mg; SERINE
530 mg; L-ASPARTIC ACID 700 mg; L-GLUTAMIC ACID 738 mg.
ELECTROLYTES (mEq/LITER): SODIUM 38 (FROM pH ADJUSTMENT);
ACETATE 72.
pH 5.8 (5.0 to 6.5)
840 mOsmol/LITER
SPECIFIC GRAVITY = 1.03
ADDITIVES MAY BE INCOMPATIBLE. CONSULT WITH PHARMACIST,
IF AVAILABLE. WHEN INTRODUCING ADDITIVES, USE ASEPTIC
TECHNIQUE, MIX THOROUGHLY AND DO NOT STORE.
SINGLE DOSE CONTAINER. CONTAINS NO BACTERIOSTAT. DISCARD
UNUSED PORTION. FOR I.V. USE. USUAL DOSAGE: SEE INSERT.
STERILE, NONPYROGENIC. STORE AT 20 TO 25°C (68 TO 77°F). [SEE
USP CONTROLLED ROOM TEMPERATURE.] AVOID EXCESSIVE HEAT. PROTECT FROM
FREEZING. AVOID EXPOSURE TO LIGHT. USE ONLY IF SOLUTION IS CLEAR AND
CONTAINER IS UNDAMAGED. MUST NOT BE USED IN
SERIES CONNECTIONS.
RX ONLY
3
V
CONTAINS DEHP
PRINTED IN USA
©HOSPIRA 2004
IM-0638 (11/04)
Hospira
HOSPIRA, INC., LAKE FOREST, IL 60045 USA
PRINCIPAL DISPLAY PANEL - 500 mL Bag Overwrap - NDC 0409-4164
TO OPEN – TEAR AT NOTCH
500 mL
NDC 0409-4164-03
Aminosyn® II
10%
Sulfite-Free
AN AMINO ACID INJECTION
Each 100 mL contains: Total amino acids approx. 10 g. Contains sodium hydroxide for pH
adjustment. Essential Amino Acids/100 mL: Isoleucine 660 mg; leucine 1000 mg; lysine (as
acetate salt) 1050 mg; methionine 172 mg; phenylalanine 298 mg; threonine 400 mg;
tryptophan 200 mg; valine 500 mg. Nonessential Amino Acids/100 mL:
N-acetyl-L-tyrosine 270 mg; alanine 993 mg; arginine 1018 mg; glycine 500 mg; proline
722 mg; histidine 300 mg; serine 530 mg; L-aspartic acid 700 mg; L-glutamic acid
738 mg. Electrolytes (mEq/Liter): Sodium 38 (from pH adjustment); acetate 72.
840 mOsmol/Liter
pH 5.8 (5.0 to 6.5)
Specific Gravity = 1.03
Single dose container. The overwrap is a moisture and oxygen barrier. Do not remove unit
from overwrap until ready for use. Visually inspect overwrap for tears or holes. Discard
unit if overwrap is damaged. Use unit promptly when overwrap is opened.
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
Protect from freezing. After removing the overwrap, check for minute leaks by squeezing
container firmly. If leaks are found, discard solution as sterility
may be impaired. COLOR VARIATION FROM PALE YELLOW TO YELLOW IS NORMAL AND
DOES NOT ALTER EFFICACY.
Rx only
7
OTHER
F WR-0292 (5/08)
Printed in USA
Hospira, Inc., Lake Forest, IL 60045 USA
Hospira

| AMINOSYN II
isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, tryptophan, valine, alanine, arginine, aspartic acid, glutamic acid, histidine, proline, serine, n-acetyl-tyrosine, and glycine injection, solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AMINOSYN II
isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, tryptophan, valine, alanine, arginine, aspartic acid, glutamic acid, histidine, proline, serine, n-acetyl-tyrosine, and glycine injection, solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AMINOSYN II
isoleucine, leucine, lysine acetate, methionine, phenylalanine, threonine, tryptophan, valine, alanine, arginine, aspartic acid, glutamic acid, histidine, proline, serine, n-acetyl-tyrosine, and glycine injection, solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Hospira, Inc. (141588017) |