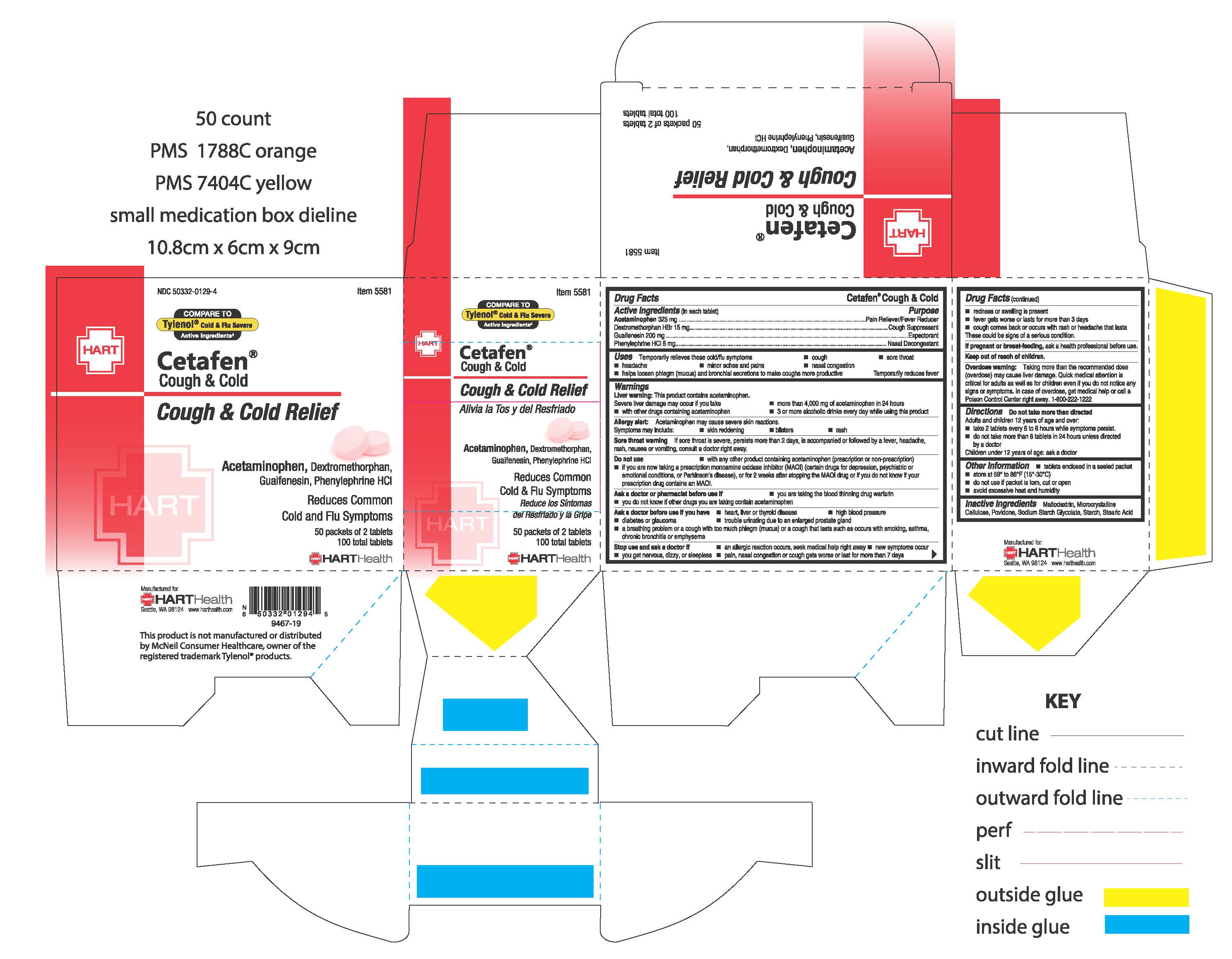
Active Ingredients (in each tablet):
Acetaminophen 325 mg
Dextromethorphan HBr 15 mg
Guaifenesin 200 mg
Phenylephrine HCl 5 mg
Uses:
Temporarily relieves these cold/flu symptoms
- cough
- sore throat
- headache
- minor aches and pains
- nasal congestion
- helps loosen phlegm (mucus) and bronchial secretions to make coughs more productive
Temporarily reduces fever
Warnings:
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions.
Symptoms may include:
- skin reddening
- blisters
- rash
Sore throat warning:
If sore throat is severe, persists more than 2 days, is accompanied or followed by a fever, headache, rash, nausea or vomiting, consult a doctor right away.
Do not use
- with any other product containing acetaminophen (prescription or non-prescription)
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI)(certain drugs for depression, psychiatric or emotional conditions, or Pakinson's disease), or for 2 weeks after stopping the MAOI drug or if you do not know if your prescription drug contains an MAO.
Ask a doctor or pharmacist before use if
- you are taking the blood thinning drug warfarin
- you do not know if other drugs you are taking contain acetaminophen
Ask a doctor before use if you have
- heart, liver or thyroid desease
- high blood pressure
- diabetes or glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem or a cough with too much phlegm (mucus) or a cough that lasts such as occurs with smoking, asthma, chronic bronchitis or emphysema
Stop use and ask a doctor if
- an allergic reaction occurs, seek medical help right away
- new symptoms occur
- you get nervous, dizzy, or sleepless
- pain, nasal congestion or cough gets worse or last for more than 7 days
- redness or swelling is present
- fever gets worse or lasts for more than 3 days
- cough comes back or occurs with rash or headache that lasts
These could be signs of a serious condition.
Overdose wasrning:
Taking more than the recommended dose (overdose) may cause liver damage. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms. In case of overdose, get medical help or call a Poison Control Center right away. 1-800-222-1222
Directions:
Do not take more than directed.
Adults and children 12 years of age and over:
- take 2 tablets every 6 to 8 hours while symptoms persist.
- do not take more than 8 tablets in 24 horus unless directed by a doctor
Children under 12 years of age: ask a doctor