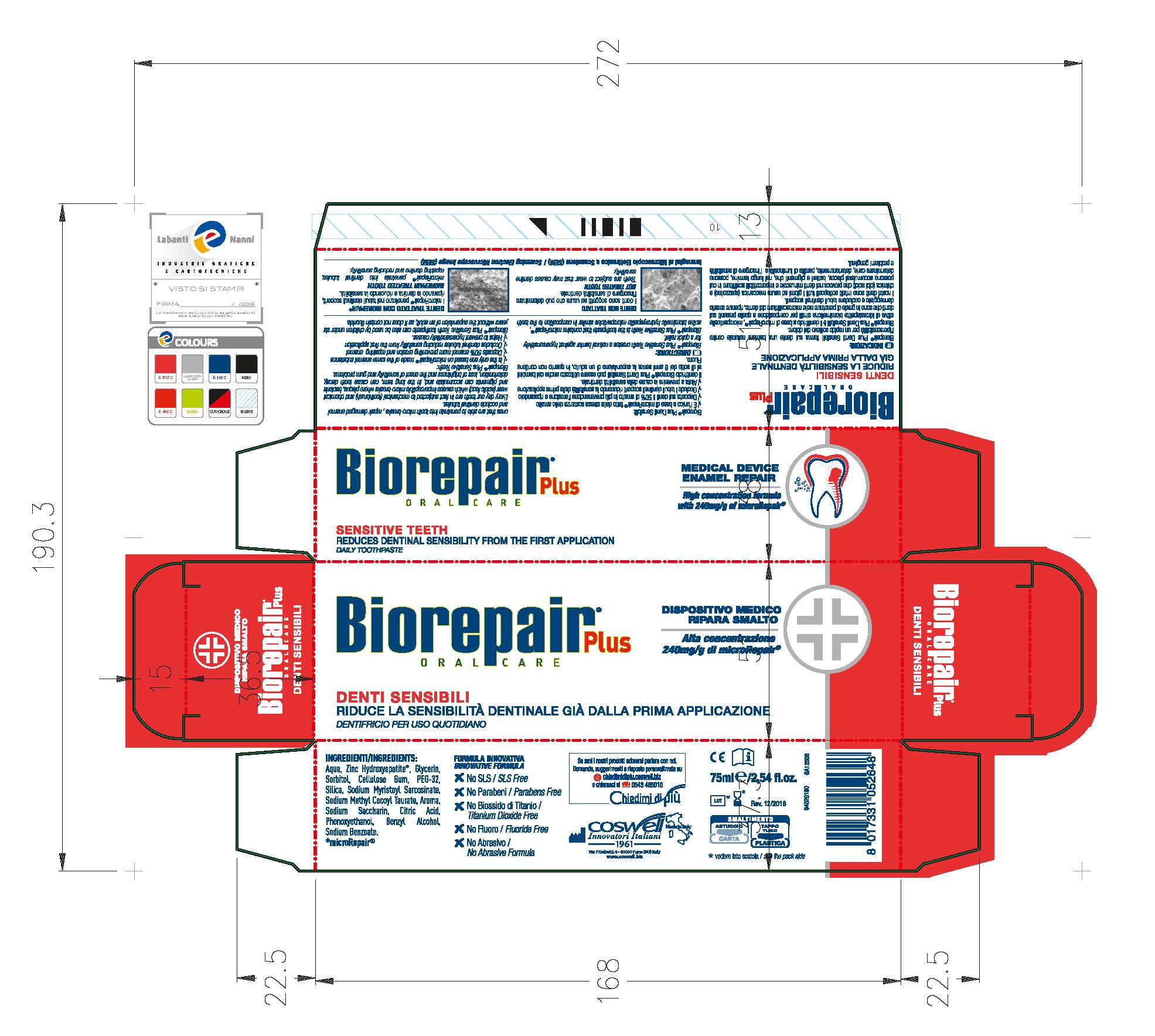
Ingredients:
Aqua, Glycerin, Sorbitol, Cellulose Gum, PEG-32, Silica, Sodium Myristoyl Sarcosinate, Sodium Methyl Cocyl Taurate, Aroma, Citric Acid, Sodium Saccharin, Benzyl Alcohol, Phenoxtethanol, Sodium Benzoate
Biorepair Plus Sensitive Teeth creates a natural barrier against hypersensitivity for a quick relief.
Biorepair Plus Sensitive Teeth is the toothpaste that contains microRepair, active biomimetic hydroxyapatite microparticles similar in composition to the teeth ones that are able to penetrate into micro-breaks, repair damaged enamel and occlude dentinal tubules.