USE(S)
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with a cold or inhaled irritants
- the impulse to cough to help your child get to sleep
- the intensity of coughing
DO NOT USE
in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
ASK A DOCTOR BEFORE USE IF YOU HAVE
- persistent or chronic cough such as occurs with asthma
- cough that occurs with too much phlegm (mucus)
STOP USE AND A ASK DOCTOR IF
- cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious illness.
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away
DIRECTIONS
- do not take more than 6 doses in any 24-hour period
- measure only with dosing cup provided
- do not use dosing cup with other products
- mL=milliliter
| Age | Dose |
| children 6 years to under 12 years | 5 mL-10 mL every 4 hours |
| children 4 years to under 6 years | 2.5 mL - 5 mL every 4 hours |
| children under 4 years | do not use |
OTHER INFORMATION
- each 5 mL contains: potassium 5 mg, sodium 5 mg
- store between 20 to 25°C (68 to 77°F)
- do not refrigerate
- dosing cup provided
INACTIVE INGREDIENTS
citric acid anhydrous, dextrose, D&C red#33, FD&C red#40, flavor, glycerin, methylparaben, potassium sorbate, propylene glycol, propyl paraben, purified water, saccharin sodium, sodium hydroxide, sorbitol, sucralose, xanthan gum
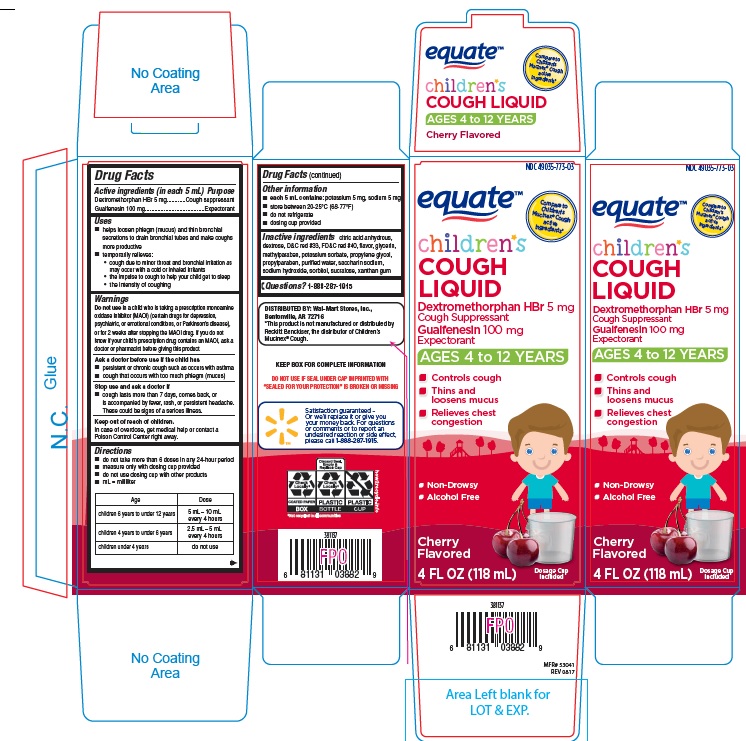
PRINCIPAL DISPLAY PANEL
NDC 49035-773-03
equate
Compare to Children's Mucinex® Cough active ingredients*
children's
COUGH LIQUID
Dextromethorphan HBr 5mg
Cough Suppressant
Guaifenesin 100 mg
Expectorant
AGES 4 TO 12 YEARS
- Controls cough
- Thins and Loosens Mucus
- Relieves chest congestion
- Non-Drowsy
- Alcohol Free
Cherry Flavored
4 FL OZ (118 mL)