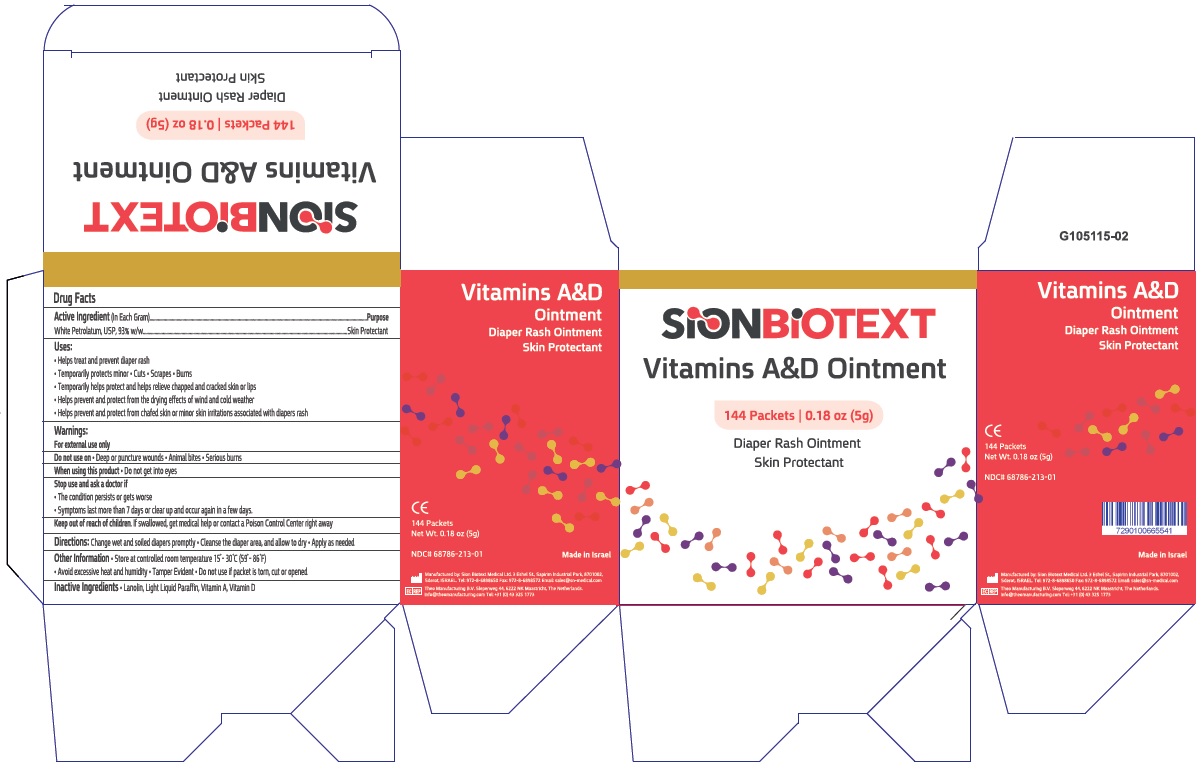
Uses
- Helps treat and prevent diaper rash
- Helps seal out wetness
- Temporarily protects minor * cuts * scrapes * burns
- Temporarily helps protect and help relieve chapped and cracked skin and lips
- Helps prevent and protect from the drying effects of wind and cold weather
- Helps prevent and protect chafed skin or minor skin irritations associated with diaper rash
- With each diaper change, especially at bedtime when exposure to wet diapers may be prolonged
Warnings:
For External Use Only
Directions
- Change wet and soiled diapers promptly
- Cleanse the diaper area and allow to dry
- Apply as needed
Other information:
- Store at controlled room temperature 15 0C to 30 0C (59 0- 86 0F)
- Avoid excessive heat and humidity
- Tamper Evident. Do not use if packet is torn, cut or open.
Principal Display panel
NDC 68786-213-01
Vitamins A&D Ointment
Intensive Moisturizer for Skin Therapy
For Personal and Professional Use
SION MEDICAL
Made in Israel
144 Packets
Net Wt. 0.17oz(5g)

NDC 68786-213-02
Vitamins A&D Ointment
Intensive Moistuirzer for Skin Therapy
For Personal and Professional Use
SION MEDICAL
Made in Israel
4 tubes
Net Wt 4 oz (113 g)

NDC 68786-213-03
Vitamins A&D Ointment
Diaper Rash Ointment
Skin Protectant
Made in Israel
12 tubes
Net Wt 4 oz (113 g)

NDC 68786-213-04
Vitamins A&D Ointment
Diaper Rash Ointment
Skin Protectant
Made in Israel
1 tube
Net Wt 4 oz (113 g)

NDC 68786-213-05
Vitamins A&D Ointment
Diaper Rash Ointment
Skin Protectant
Made in Israel
144 Packets
0.18 oz (5 g)