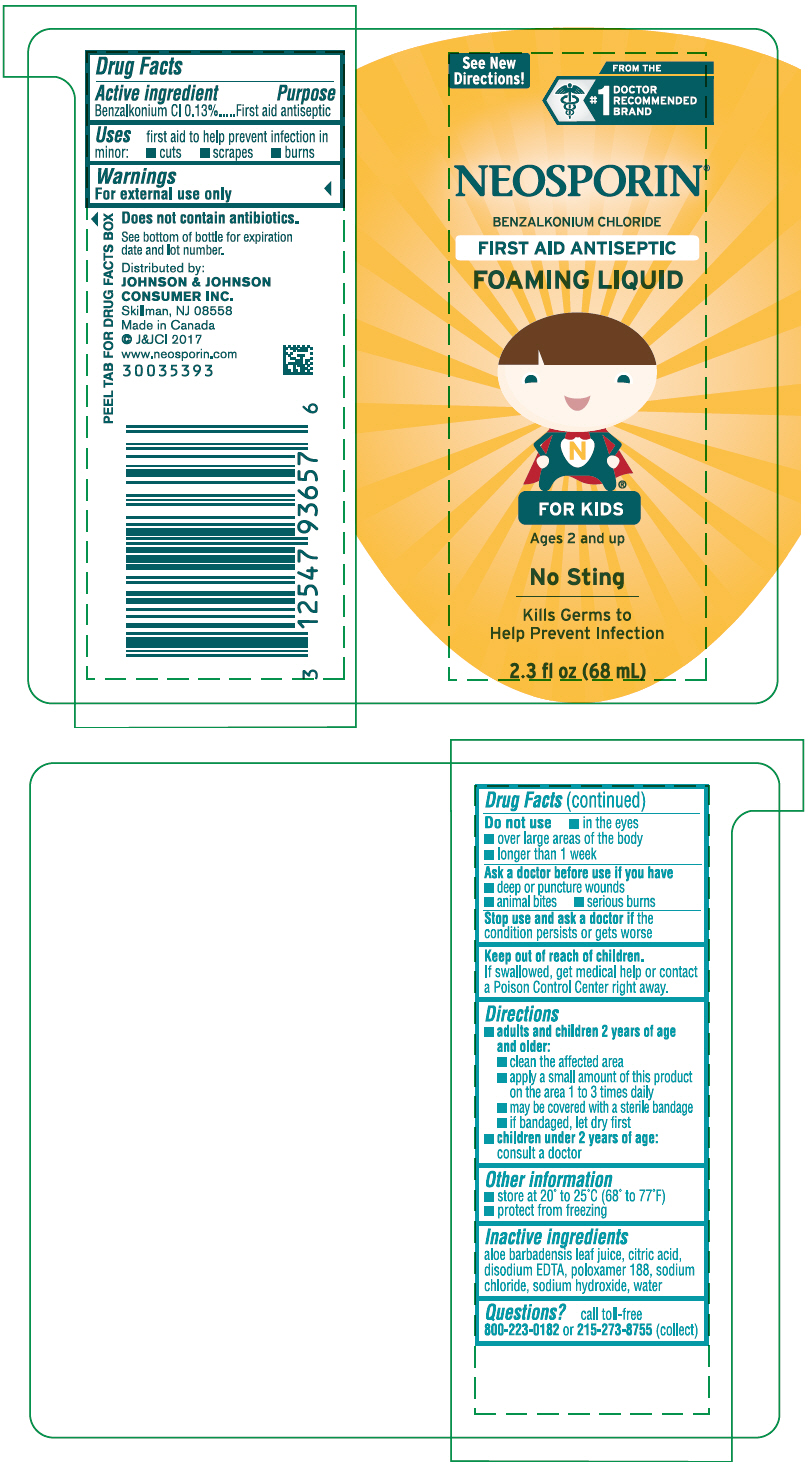
NEOSPORIN FIRST AID ANTISEPTIC FOAMING FOR KIDS- benzalkonium chloride liquid
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
NEOSPORIN ® FIRST AID ANTISEPTIC FOAMING LIQUID FOR KIDS ®
Directions
-
adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product on the area 1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
-
children under 2 years of age:
consult a doctor
| NEOSPORIN FIRST AID ANTISEPTIC FOAMING FOR KIDS
benzalkonium chloride liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (118772437) |
Revised: 1/2023
Document Id: ee484505-3bb3-2989-e053-2995a90aea2b
Set id: 5018028a-00b6-46e5-ac12-103b7c5213a6
Version: 4
Effective Time: 20230112
Johnson & Johnson Consumer Inc.