Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product,
do not take more than directed. Taking more than directed may cause drowsiness.
Directions
| adults and children 6 years and over | 1 tablet daily; not more than 1 tablet in 24 hours |
| children under 6 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
Other information
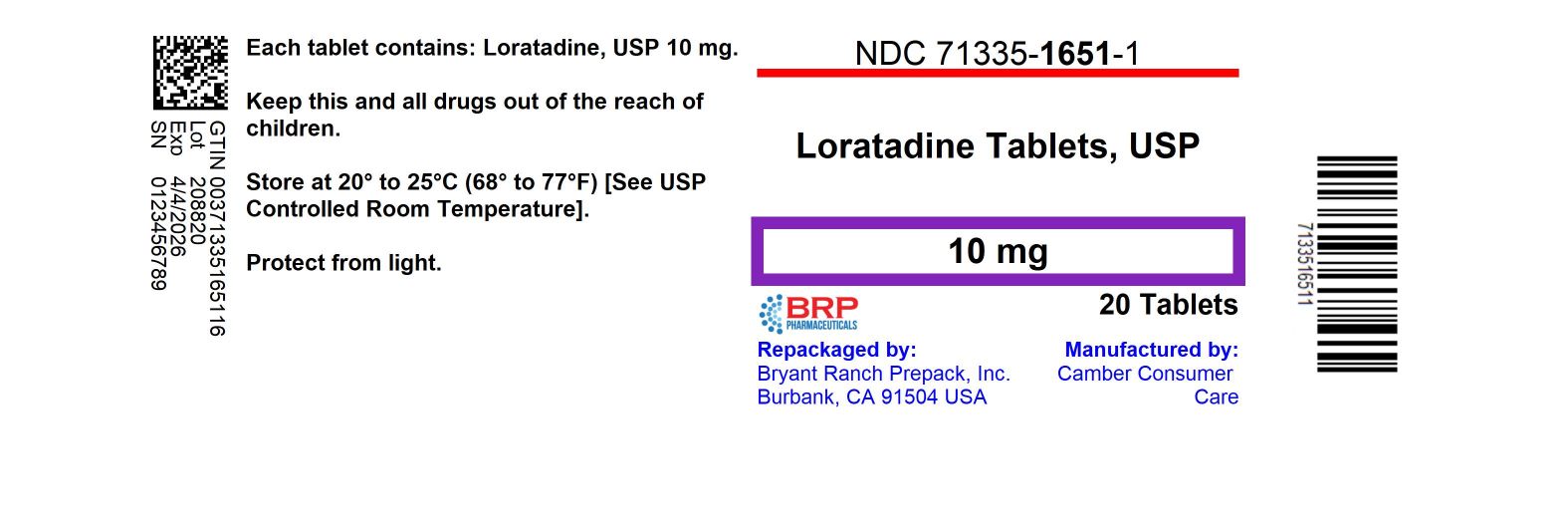
- store at 20º-25ºC (68º-77ºF) (see UPS Controlled Room Temperature)
- protect from light
Inactive ingredients
lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium starch glycolate
HOW SUPPLIED
NDC: 71335-1651-1: 20 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1651-2: 30 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1651-3: 60 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1651-4: 14 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1651-5: 10 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1651-6: 90 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1651-7: 28 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1651-8: 15 Tablets in a BOTTLE, PLASTIC
NDC: 71335-1651-9: 100 Tablets in a BOTTLE, PLASTIC